Background: Antiphospholipid syndrome (APS) is characterized by the development of thromboembolic events in the setting of persistent antiphospholipid antibodies (APA). While several studies have investigated the clinical characteristics and venous thromboembolic (VTE) outcomes of pediatric patients with a diagnosis of APS, there is a scarcity of published data describing the incidence, natural history and VTE outcomes of children who present with positive APA at the time of an acute VTE episode.
Objective: To describe the incidence, natural history and outcomes of transient and persistent APA in patients ≤21 years old with a first episode of provoked VTE.
Methods: Patients with positive APA at the time of enrollment in an ongoing NHLBI-sponsored multicenter, parallel-cohort randomized controlled trial (RCT) on duration of anticoagulation therapy in provoked VTE (the Kids-DOTT Trial; NCT00687882) were included in this analysis. APA testing was obtained at the time of enrollment in all patients, and at 6 weeks post-VTE diagnosis in those with an initial positive APA result. Subsequent follow-up testing in patients whose APA persisted at 6 weeks post-VTE was performed at the discretion of the enrolling site's treating hematologist. Patients with persistent APA at 6 weeks were treated in a non-randomized parallel-cohort of the trial in which patients received at least a 3-month therapeutic course of anticoagulation. Those without persistent APA at 6 weeks were retained in the RCT and randomized to shortened-duration (no further therapy) versus conventional duration (total duration = 3 months) of therapy. Measured APA included lupus anticoagulant-sensitive activated partial thromboplastin time (LAS-aPTT), dilute Russell viper venom time (DRVVT), hexagonal phase phospholipid assay (STACLOT LA), anti-cardiolipin (ACL) IgM, and anti-beta-2-glycoprotein-1 (aß2GPI) IgG and IgM. Data were prospectively collected. Descriptive statistics were used to summarize data on demographic characteristics, VTE presentation, and outcomes of interest (persistent thrombus occlusion at 6 weeks post-VTE, post-thrombotic syndrome [PTS], recurrent VTE and clinically-relevant bleeding [CRB]). The blind was maintained in the Kids-DOTT trial throughout data transfer and analysis.
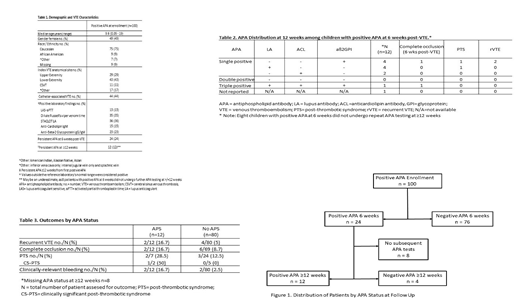
Results: APA testing was performed in 476 patients at the time of enrollment. Twenty-one percent of patients (n=100) had at least one positive APA and were included in the present analysis. Demographics, VTE characteristics, and laboratory findings at enrollment are summarized in Table 1. Median age was 9.6 years (range 0.05-19). The most common thrombosis site was the lower extremity (43%). Presence of a central venous catheter was the provoking factor in 44% of cases. LA was the most common APA detected at the time of enrollment (36%, 35% and 13% of patients by STACLOT, DRVVT and LAS-aPTT, respectively). Twenty-four percent of patients had a persistent APA at 6 weeks post-VTE (Figure 1). Of these, 16 (66.7%) had repeat APA testing at least 12 weeks from first positive APA. Persistent APA at ≥12 weeks (i.e., APS) was determined in 12 of the 16 patients. Most of these children with APS had a single positive APA (aß2GPI IgG/IgM, n=4, LA, n=4, ACL IgM, n=2) while only 1 patient had triple-positive APA (Table 2). Rates of recurrent VTE, complete venous occlusion at 6 weeks post-VTE, development of PTS, and CRB were higher in APS patients as compared to those without APS (Table 3).
Conclusions: This Kids-DOTT analysis reports for the first time that positive APA are found in nearly one quarter of children and young adults with provoked VTE at the time of the acute VTE episode, and that approximately 75% of these are transient (negative at 6 weeks post-VTE diagnosis). Patients with provoked VTE who have APS tended to have increased risk of adverse VTE outcomes when compared to those without APS; however, the size of these subgroups does not permit a definitively-powered comparison in outcomes. The putative role of persistently positive APAs as a prognostic factor for adverse VTE outcomes should be formally tested upon completion of the Kids-DOTT trial.
Tarango:Shire: Membership on an entity's Board of Directors or advisory committees; Bayer: Other: Study steering committee. Goldenberg:NIH: Other: research support and salary support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal