
Introduction: Severe vaso-occlusive pain episodes (VOE) are a major cause of morbidityand mortality in sickle cell anemia (SCA). Low arginine bioavailability is associated with pain severity and predicts need for pediatric hospitalization (Morris et al, 2000). Arginine supplementation has opioid-sparing effects and was found to significantly decrease pain scores in children hospitalized with SCA-VOE compared to placebo in a phase-2 randomized placebo-controlled trial (RCT) performed in the United States (US, Morris et al, 2013). Its role to treat acute SCA-related pain in a Sub-Saharan African country is unknown.
Objectives: To determine the role of oral arginine as adjuvant in the management of SCA-VOE.
Methods: A double-blind RCT of oral L-arginine (100 mg/kg/dose every 8 hours until discharge; up to 5 days/15 doses maximum) was performed in children with SCA hospitalized with severe VOE defined as a Numerical Pain Scale Score (PS) of at least 7 on a scale of 0-10, at one of two hospitals in Abuja, Nigeria. All patients received pain management (both opioids and non-opioid analgesics) per institutional practice. Demographics, clinical characteristics, length of hospital stay (LOS), pain scores, time-to-crisis-resolution (time-to-PS<4), analgesia medications required, and plasma amino acids levels were obtained before and after supplementation. Mean total analgesic medication quantification scale score (MQS), a sensitive measure to quantify analgesic use in patients with SCA, was utilized as previously described (Jacob et al, 2007). Opioid doses were calculated based on morphine equivalents in milligrams (mg).
The study was performed after obtaining the National Agency for Food & Drug Administration & Control (NAFDAC) approval, Ref No. NAF/DER/CT/LAG/2017. The protocol was approved by the Human Research Ethics Committees of the University of the Witwatersrand, University of Abuja Teaching Hospital, Abuja & the National Hospital. The study was registered at the Pan African Clinical Trial Registry (PACTR 201611001864290).
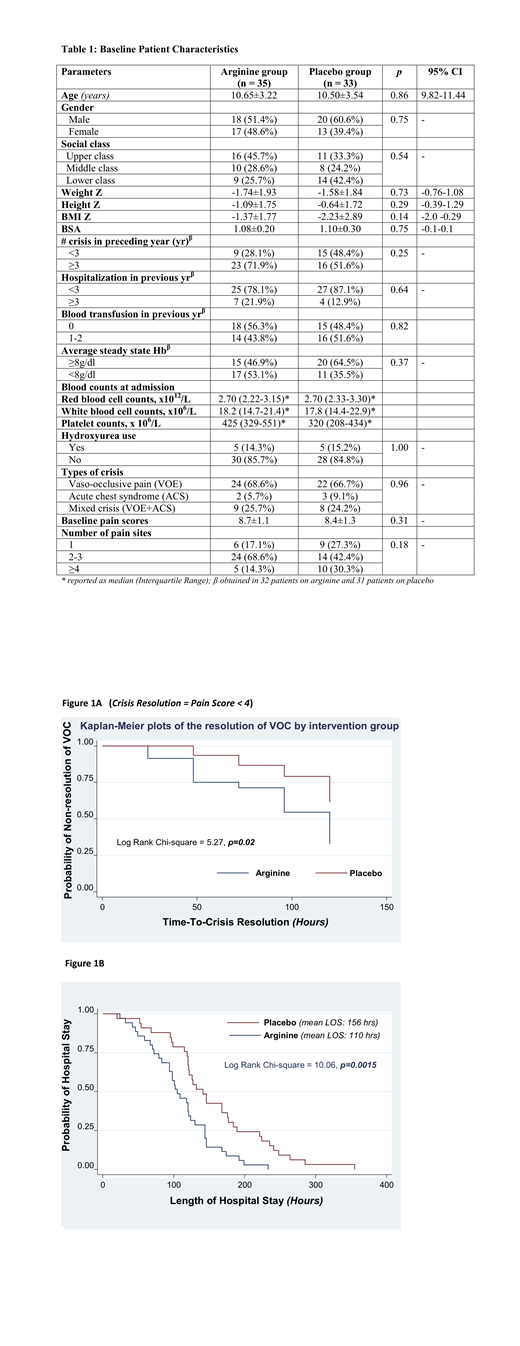
Results: Sixty-eight children with SCA were recruited, aged 5-17 years (mean: 10.6±0.4 years; 35 children randomized into the arginine arm & 33 into the placebo arm). Baseline characteristics were similar between arms (Table 1). MQS was significantly lower in the arginine group vs. placebo (73 [95% CI: 62-84] vs. 120 [97-143]; p<0.001). By day 5, 54% of children treated with arginine had been discharged compared to 24% in the placebo arm. Although PS were similar in both groups prior to study drug delivery (8.7±1.1 vs. 8.4±1.2, arginine vs placebo; p=0.30), worst reported PS on day 5 were lower in children treated with arginine compared to placebo (1.2±0.4 vs. 3.0±0.5; p<0.0001). The mean rate of PS decline was also greater in the arginine arm vs. placebo (1.5 [1.2-1.8) vs. 1.1 [0.9-1.2] cm/day; p=0.009). Plasma arginine levels increased by 125% vs 29% in the arginine arm vs. placebo; percent increase in arginine bioavailable inversely correlated with MSQ (r=-0.35;p=0.02). There was a non-statistically significant decrease in mean total opioid dose used in the arginine group vs. placebo (3.8 [2.7-4.9] vs 5.1 [3.8-6.5) mg/kg, p = 0.11). Patients receiving arginine had shorter time-to-crisis-resolution (p=0.0216, Fig1A), shorter LOS (p=0.015; Fig1B) and had no serious adverse events. There was 1 death in the placebo group on the second day of admission. There were no differences between groups in development of adverse events, however there was a trend towards more vomiting in the arginine arm compared to placebo (20% vs. 3%, p=0.07).
Conclusion: Arginine deficiency plays a role in acute pain requiring hospitalization in Nigerian children with SCA, similar to what has been reported in the US. Plasma arginine levels significantly increased with arginine supplementation, and improved global arginine bioavailability was inversely associated with total analgesia and opioids used in VOE management. Total mean analgesia use and pain scores were lower, while time-to-crisis-resolution and LOS were shorter in children treated with arginine compared to placebo. No serious adverse events occurred in the arginine arm, while rates of adverse events were similar to placebo, providing further support for the safety of arginine therapy in children with SCA. Oral arginine is a promising adjuvant therapy for SCA-VOE management.
Morris:Pfizer: Consultancy; UCSF-Benioff Oakland: Patents & Royalties: Patents owned by UCSF-Benioff Children's Hospital Oakland regarding biomarkers and therapies that target arginine bioavailability; none are licensed and there is no royalties generated; UCSF-Benioff Children's Hospital Oakland: Patents & Royalties: Patents owned by UCSF-Benioff Children's Hospital Oakland, licensed by Lifetrients, generating royalties for nutritional supplement for autism/apraxia.
Oral L-arginine for treatment of acute sickle cell disease vaso-occlusive pain
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal