Background: CD19-targeted chimeric antigen receptor T cell therapy (CART19) has demonstrated remarkable clinical efficacy in treating relapsed/refractory B cell ALL, but associated toxicities may require treatment in inpatient or intensive care units (ICU). We sought to: (1) describe inpatient and ICU resource utilization within 30 days of CART19 infusion; and (2) evaluate trends in resource utilization from 2012-2019.
Methods: We identified patients treated with CART19 on a clinical trial (NCT01626495, NCT02906371, and NCT02374333) or with the commercial product, tisagenlecleucel, at Children's Hospital of Philadelphia. Patients who received a prior cell therapy product were excluded. Demographic, pharmacy, and inpatient data were extracted from the electronic medical record from day of infusion (day 0) to day +30, censored at disease progression or death, using a semi-automated EPIC data query tool (ExtractEHR). The Virtual Pediatric Systems (VPS) database was queried for clinical data, resource utilization data, and Pediatric Risk of Mortality (PRISM) 3 and Pediatric Index of Mortality (PIM) 2 severity of illness scores. Log-binomial regression and linear regression were used to estimate the association of patient characteristics with the need for inpatient/ICU admission and inpatient/ICU length of stay (LOS), respectively. Similar models were used to estimate trends in outcomes from 2012-2019.
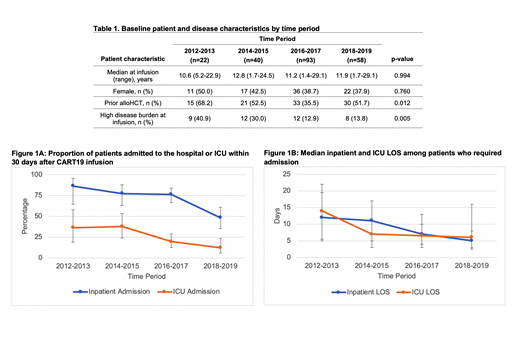
Results: A total of 213 patients were included in the analyses. Median patient age was 12.4 years (range 1.4-29.1) at infusion; 60% were male, 66% were Caucasian, and 80% were non-Hispanic. Prior to CART19, 42% had an allogeneic hematopoietic cell transplant (alloHCT). At time of infusion, 19% had high disease burden, defined as bone marrow blasts ≥40% by flow cytometry. From 2012-2019, the proportion of patients with prior alloHCT or high disease burden decreased (Table 1).
CART19 was infused in the outpatient setting in 93% of patients. In the 30 days after infusion, 70% had at least one inpatient admission, starting at a median of day +2 (IQR +1 to +6). Among the 149 patients admitted, median cumulative inpatient LOS was 7 days (IQR 4-13). Cumulative LOS increased with increasing grade of cytokine release syndrome (CRS). Median LOS was 0, 5, and 15 days for patients with no, mild, and severe CRS, respectively. From 2012-2019, there were linear trends toward decreases in proportion of patients admitted (p<.0001) and in cumulative inpatient LOS (p=0.001, Figure 1), which remained significant after adjustment for disease burden (p=0.032 and p<.0001).
ICU admission was required for 23% (95% CI, 17-29) of the cohort, starting at a median of day +5 (IQR +4 to +6.5). ICU admission was more frequent for patients with high disease burden than those with low disease burden [68% (95% CI, 52-81) vs. 11% (95% CI, 7-17), p<.0001]. Among the 48 patients admitted to the ICU, resources utilized included vasoactive agents (n=36, 75%), non-invasive or invasive mechanical ventilation (n=24, 50%), and renal replacement therapy (RRT; n=4, 8%). In the 30-day follow-up period, median ICU LOS was 7 days (IQR 3-11.5), but 6 (12.5%) patients remained in the ICU after day +30. Among patients who required vasoactives, invasive mechanical ventilation, or RRT, the median duration of resource utilization was 5, 7.2, and 2 days, respectively. Seventy five percent (n=36) of patients admitted to the ICU received tocilizumab. Predicted median risk of mortality was 6.02% (IQR 5.04-7.32%) by PIM 2 and 10.74% (IQR 6.78-27.08%) by PRISM 3 scores. However, observed 30-day mortality was 1% (n=2) across the cohort, or 4% among ICU patients. From 2012-2019, there was a decrease in proportion of patients requiring ICU admission (p=0.001), but no significant changes in cumulative ICU LOS (Figure 1).
Other than high disease burden, baseline patient characteristics were not significantly associated with inpatient/ICU admission or inpatient/ICU LOS.
Conclusion: In a cohort of 213 pediatric patients with ALL who received CART19, over 90% were safely infused in the outpatient setting. Though the majority of patients required at least one inpatient admission, the proportion of patients admitted to the hospital or ICU and cumulative inpatient LOS in the 30 days post-infusion decreased over the past 7 years. Mortality was 1%. Additional analyses will investigate the impact of changes in supportive care practices on resource utilization outcomes.
Grupp:GSK: Consultancy; Cure Genetics: Consultancy; Humanigen: Consultancy; CBMG: Consultancy; Novartis: Research Funding; Kite: Research Funding; Novartis: Consultancy, Research Funding; Roche: Consultancy; Servier: Research Funding; Jazz: Other: study steering committees or scientific advisory boards; Adaptimmune: Other: study steering committees or scientific advisory boards. Maude:Novartis: Consultancy; Kite: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal