Background:
Clinical trials, key elements of the processes that account for many of the recent advances in cancer care, are becoming more complex and challenging to conduct. The Stephenson Cancer Center (SCC) has been the lead accruer to NCI-LAP trials over the past three years, and in addition, fields investigator initiated and industry sponsored trials. To identify opportunities for continued improvement in clinical trial enrolment, we sought to identify the obstacles encountered by our clinical trial staff in these activities.
Method:
We conducted a survey of our research staff including all research nurses and disease site coordinators who participate in recruitment, screening, consenting, data collection and compliance. The survey, sent by email to the clinical trial list-serve at SCC (90 staff member), invited respondents to enumerate obstacles to patient participation in clinical trials.
We then performed a follow up meeting with our research coordinators to clarify responses. A total of 26 responses from 90 respondents were received and tabulated by disease site.
Results:
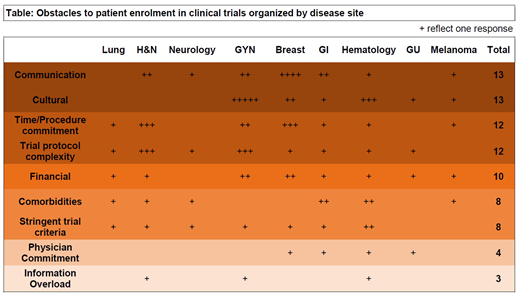
The most commonly reported obstacles to enrolment were, in descending order: communication/language barriers, cultural bias, time/procedure commitment, and complexity of the trial protocol, financial logistics, comorbidities, and stringent trial criteria. Respondents identified 83 obstacles as frequently encountered obstacles to enrolment. The 83 reported obstacles were classified into 9 categories and organized by disease site as presented in tabular format (below).
The most commonly identified obstacles to patient enrolment were communication and language barriers. In patients for whom Spanish is the primary language this was a universal obstacle, as there is a lack of consistent Spanish consents across the clinical trial portfolio. Cultural bias, as an obstacle was manifested as a general mistrust by prospective trial participants of experimental therapies and clinical trials. After communication and cultural bias as barriers, travel requirements and the associated expenses playing a role in patients from rural areas were identified as the most commonly encountered barrier.
The complexity of trial protocols and the associated large number of clinic visits, frequent laboratory and imaging tests were also identified as common obstacles. Clinical trial complexity with strict inclusion and exclusion criteria and trial-specified biopsies were frequently cited.
Implications:
In this descriptive study, common barriers to patient enrolment in clinical trials were identified by clinical trial staff. Assessing barriers encountered by clinical trial staff is infrequently used as a metric for improving clinical trial enrolment, but provides important perspective. In our study, some obstacles are inherent in our patient populations, others appear to be actionable. Development of Spanish language consents and specific programs to overcome negative bias regarding clinical trials are potential areas for improvement. The complexity of clinical trial protocols and the increasingly strict inclusion/exclusion criteria, are issues that will require consideration and action at the level of the cooperative groups and industry.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal