Background: Patients with BCR-ABL-negative myeloproliferative neoplasms (MPNs) have high symptom burdens that negatively impact quality of life, including risk for developing chronic pain and psychosocial complications. The NCCN guidelines recommended the use of the MPN Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) starting in 2017 to assess symptom burden. Our primary aim was to determine if the MPN-SAF TSS has been incorporated into patient care, and if not, how well BCR-ABL-negative MPN symptoms are captured by review of systems (ROS). Our secondary aim was to evaluate the prevalence of anxiety, depression, chronic pain, and opiate use in our patient population.
Methods: We performed a single-center, cross-sectional study of all active BCR-ABL-negative MPN patients in the UCSF Hematology Clinic between January 2017 and March 2019. We reviewed all hematology visits for completion of the MPN-SAF TSS, and the most recent visit for the number symptoms from the MPN-SAF TSS explicitly captured in ROS or problem list and relevant medications. Descriptive statistics were used to summarize the data. Patients whose disease transformed into acute leukemia or were post-allogeneic stem cell transplantation were excluded.
Results: Of 299 patients with BCR-ABL-negative MPN diagnoses, the median age was 66 (range 20-98) with an equal number of males (n=150) and females (n=149). Essential thrombocythemia (ET) was the most common diagnosis (n=109; 37%), followed by polycythemia vera (PV) (n=90; 30%), primary myelofibrosis (MF) (n=49; 16%), post-PV or post-ET MF (n=29; 10%), and MPN-Unclassifiable or overlap (n=22; 7%). Most were JAK2 V617F positive (n=213; 71%) and high-risk (n=148; 49.5%) by clinical criteria, IPSET-thrombosis, and DIPSS-plus. Nearly all were on active treatment (91%), with aspirin (n=205; 69%), hydroxyurea (n=130; 43.5%), phlebotomy (n=61; 20%), and ruxolitinib (n=37; 12%) being the most frequent treatments. Significant disease-related vascular complications were documented in 20.7% of patients.
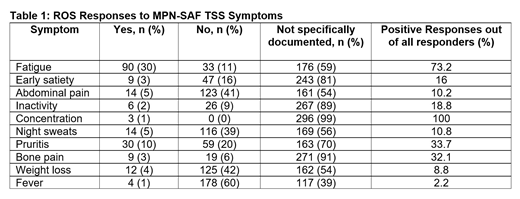
The 299 patients were evaluated by 22 hematology providers. The MPN-SAF TSS was formally documented in 1 patient (0.3%). Of the 10 symptoms in the MPN-SAF TSS, the median number documented as positive or negative on ROS was 3 (range 0-8), with 0 or 1 symptoms documented in 82 patients (27.4%). The mean number of positive symptoms was 0.7 (range 0-4) with at least 1 positive symptom reported by 44.7%. The most frequently charted symptoms were fever (n=182; 60.9%), unintentional weight loss (n=137; 45.8%), abdominal pain (n=137; 45.8%), and night sweats (n=130; 43.5%). More unique MPN symptoms were documented less frequently, including pruritis (n=89; 29.8%), early satiety (n=56; 18.7%), bone pain (n=28; 9.4%), and problems with concentration (n=3; 1%). The most common positive symptoms were fatigue (n=90; 73%), pruritis (n=30; 33.7%) and bone pain (n=9; 32%).
Pain and psychological symptoms were infrequently charted in hematology clinic. Pain medications were used by 20.4% with nearly half (48%) on opiates, but chronic pain was on the provider problem list for only 5.7% of patients. Anxiety/depression medications were used by 20.4%, but anxiety/depression was on the provider problem list in only 4% of patients.
Conclusions: To our knowledge, this is the first study to assess the implementation of the MPN-SAF TSS into clinical practice since the NCCN recommendations went into effect. The MPN-SAF TSS is not being utilized in regular practice at our site and a low number of disease-related symptoms are documented in clinic notes. Fatigue is the predominant symptom in our patients, which is similar to previously published studies. The discrepancy between medications taken and symptoms documented suggests that patients have a higher symptom burden than reported. Implementing a standardized way of consistently capturing patients' MPN-related symptoms in hematology practice will be explored in future quality improvement research.
Damon:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Logan:Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Research Funding; Kite: Research Funding; Kadmon: Research Funding; Pharmacyclics: Research Funding; Novartis: Consultancy; TeneoBio: Consultancy; Kiadis: Consultancy; Astellas: Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mannis:Jazz: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Abbvie/Genentech: Membership on an entity's Board of Directors or advisory committees; Forty Seven: Membership on an entity's Board of Directors or advisory committees; Curis: Membership on an entity's Board of Directors or advisory committees. Olin:Spectrum: Research Funding; Novartis: Research Funding; Mirati Therapeutics: Research Funding; MedImmune: Research Funding; Ignyta: Research Funding; Clovis: Research Funding; Daiichi Sankyo: Research Funding; Astellas: Research Funding; Genentech: Consultancy, Research Funding; Pfizer: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria; Revolution Medicine: Consultancy; AstraZeneca: Research Funding. Shah:Bristol-Myers Squibb: Research Funding. Atreya:Immunotherapeutics: Honoraria; Guardant Health: Research Funding; Novartis: Research Funding; Merck: Research Funding; Kura Oncology: Research Funding; Array Biopharma: Honoraria; Pionyr: Honoraria; Bristol-Meyers Squibb: Research Funding. Smith:Astellas Pharma: Research Funding; Abbvie: Research Funding; fujiFilm: Research Funding; Revolution Medicines: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal