Introduction: Neurologic adverse events remain challenging complications with poor morbidity and mortality post adult allogeneic hematopoietic cell transplantation (alloHCT) for hematologic diseases. We conducted a systematic review and meta-analysis to determine their spectrum, incidence and impact on survival.
Methods: We searched MEDLINE, COCHRANE, EMBASE through March 2019 for studies published in English. We deemed eligible all types of primary studies including randomized controlled trials (RCTs), controlled observational studies (both cohort and case control), case series and case reports. The eligibility criteria were: (a) adult population specified as patients fifteen years old or older, (b) alloHCT for hematologic diseases, (c) neurologic disorders' diagnosis. Two independent reviewers screened, extracted data and assessed risk of bias (RoB).
The primary outcome being the incidence of neurologic complications was assessed in a form of a proportion of adult patients with the neurologic manifestation (n) from the total group of patients that received alloHCT for hematologic diseases (N). We performed a random-effects meta-analysis of proportions using the Freeman-Tukey double arcsine transformation, including only cohort studies. Secondary outcome was impact of neurologic complications on overall survival of patients presented with symptoms after allo-HCT.
Pre-planned sensitivity analysis integrating the risk of bias assessment by excluding studies evaluated as high risk was performed as well as prespecified subgroup analyses according to the type of neurologic event.
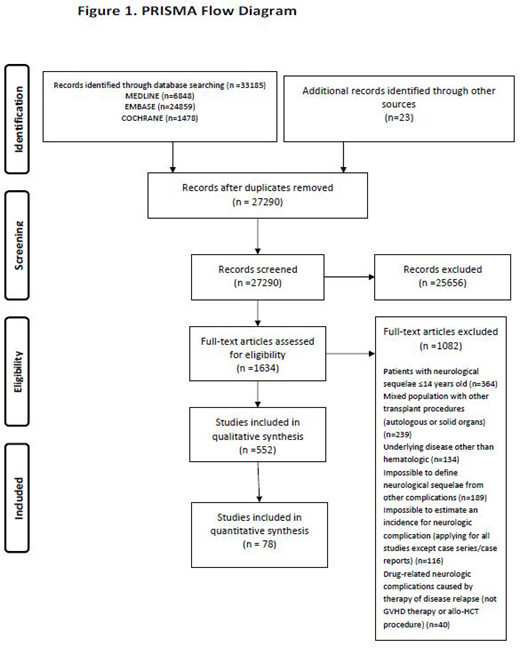
Results: We identified 552 eligible studies describing 57.972 patients; one randomized controlled trial, two case-control, 17 prospective, 86 retrospective cohort studies, 21 case-series and 425 case-reports (PRISMA flow diagram). RoB ranged from fair to high although case-series were of low-risk. The majority of studies traced infectious or drug-related neurologic manifestations. Incidence rates of neurologic complications varied, according to type of complications and studies, from 0.6% for immune-mediated disorders in retrospective cohorts to 13% for drug-related events in prospective cohorts.
In meta-analysis of proportions, we included only cohort studies, retrospective or prospective, with homogenous population to estimate a proportion. Neurologic clinical signs or symptoms were detected in 1415 out of 37450 [6.2% (95%CI 4.8-7.7), Ι2= 96.1% (p<0.001)] patients described in 78 included cohort studies. Heterogeneity of results remained high, even after sensitivity analysis excluding studies of high RoB. Infectious complications were present in 2.7% (95%CI 1.9-3.6) and 3.3% (95%CI 0.8-7.1) of patients in retrospective and prospective cohort studies respectively. In retrospective studies, 3.4% (95%CI 2.1-4.9) of patients suffered from drug-related neurologic events. In prospective cohorts, the equivalent incidence was 13% (95%CI 4.2-24.8). Other neurologic complications included cerebrovascular events, thrombotic microangiopathy, immune-mediated complications, relapse and metabolic events.
Regarding the severity of neurologic complications and death rate in patients with outcome of interest, data were too diverse to provide any safe conclusion. Neurologic complications had a detrimental impact on survival depending on type of complication in various studies.
Based on study type, high RoB in most of the included studies, as well as significant heterogeneity in results from observational data, high imprecision and suspected publication bias, quality of evidence was very low applying the GRADE tool.
Conclusion: Our study highlights the wide spectrum and significant impact of neurologic complications on survival post alloHCT. This systematic review summarizes existing data and provides the necessary background information for every physician involved in the management of these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal