Introduction: Graft failure remains a major obstacle to the success of allo-HSCT. Rates of graft failure are reported to be 2% in recipients of HLA identical grafts, 9% in those with an unrelated donor, and up to 12.3% in those with an HLA-mismatched related donor (0-3 antigen mismatched). Graft failure is associated with high mortality due to prolonged cytopenia's leading to significant anemia, bleeding, and infection. Successful management of graft failure usually requires a second transplant to restore normal hematopoiesis. The selection of a preparative regimen presents a challenge in these patients who have recently undergone myelosuppressive therapy prior to the failed transplant maneuver. We have employed the use of a minimally myelosuppressive regimen consisting of a single dose of pentostatin with a low dose of total body irradiation (TBI) as conditioning prior to a second allogeneic transplant for patients who experienced primary graft rejection.
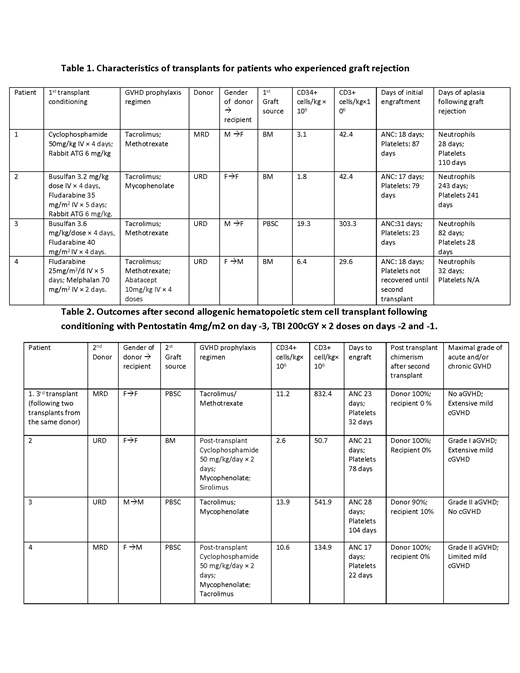
Methods: Under an IRB-approved retrospective study, we describe 4 consecutive patients with graft failure after allogeneic stem cell transplantation, who received a re-conditioning regimen consisting of pentostatin and low-dose TBI prior to second allo-HSCT. The median age of patients was 38 years (23-51). The conditioning regimens of the first transplant were myeloablative doses of cyclophosphamide/rabbit ATG; busulfan/fludarabine rabbit ATG/; busulfan/fludarabine; and fludarabine/melphalan (Table 1). Median cell doses for the first transplant were 7.7 x 10E6 CD34+ cells/kg and 104 x 10E6 CD3+ cells/kg. Two patients had primary graft rejection and 2 patients had secondary graft rejection. Patients with secondary graft rejection had initial hematopoietic engraftment of neutrophils on a median of 21 days and platelets on a median 61 of days post-transplant with subsequent declines in peripheral blood counts and chimerism studies showing loss of donor cell engraftment. The donor for the second transplant was the same in one patient and different in three patients and included a HLA-matched sibling in 2/4 patients and HLA-matched unrelated donor (URD) in 2/4 patients. One patient had two transplants from the same donor followed by a third transplant from a different HLA matched sibling donor. Conditioning for the second transplant in these cases consisted of pentostatin 4 mg/m2 as a single dose on day -3 and two fractions of 200 cGy TBI on days -2 and -1 as shown in Table 2.
Results: Patients underwent the second (or third) donor allogeneic transplant at a median of 99 days following the first transplant. The second donor allograft contained a median of 9.6 x 10E6 CD34+ cells/kg and 390 x 10E6 CD3+ cells/kg. All recipients of the pentostatin-TBI conditioning regimen engrafted following transplantation of allogeneic stem cells (Table 2). Engraftment following the second transplant (or third transplant, in patient #1) occurred at a median of 22 days for neutrophils and 54 days for platelets, and all patients achieved 100% myeloid and lymphoid chimerism. Patients did not experience VOD/SOS or have severe mucositis, enteritis, or pulmonary toxicity, and were hospitalized a median of 39 days from the second transplant. All four patients achieved normal blood counts following the second transplant. One patient died unexpectedly of an apparent infection following full donor hematopoietic reconstitution. Three of the four patients undergoing a second transplant using pentostatin/TBI conditioning are alive without evidence for disease relapse or graft rejection at a median follow-up of 3.5 years, with none of these patients experiencing severe acute or chronic GvHD.
Conclusion: Single dose pentostatin combined with low-dose TBI represents an effective and well-tolerated conditioning regimen that facilitates engraftment of a second allogeneic transplant in patients who experienced primary or secondary graft rejection. The enhanced therapeutic efficacy of this reduced-intensity regimen that allowed consistent donor-derived hematopoietic engraftment after initial allo-graft rejection warrants further study.
Langston:Astellas Pharma: Other: Research Support; Incyte: Other: Research Support; Jazz Pharmaceuticals: Other: Research Support; Chimerix: Other: Research Support; Takeda: Other: Research Support; Kadmon Corporation: Other: Research Support; Novartis: Other: Research Support; Bristol Myers Squibb: Other: Research Support. Waller:Pharmacyclics: Other: Travel expenses, Research Funding; Cerus Corporation: Other: Stock, Patents & Royalties; Chimerix: Other: Stock; Cambium Oncology: Patents & Royalties: Patents, royalties or other intellectual property ; Amgen: Consultancy; Kalytera: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
the use of pentostatin as part of a conditioning regimen
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal