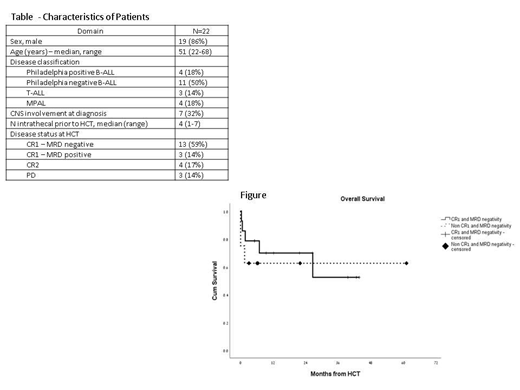
Introduction: Acute lymphoblastic leukemia (ALL) and mixed phenotype acute leukemia (MPAL) in adults are associated with a cure rate of only 20 and with a tendency for relapse to the CNS. Total body irradiation (TBI)-based high dose therapy and allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for these patients, however, the median age for ALL in adults is 65 years and thus, majority of patients are not suitable candidates for high dose conditioning and HCT. Therefore, novel approaches are needed. Thiotepa is an alkylating lipophilic agent that result in achieving up to 100% cerebrospinal fluid levels and has been used in ablative regimens (total dose of 20 mg/kg), Christopoulos BBMT 2012. Methods: We aimed to test this regimen in patients with ALL/MPAL who were not eligible for a standard TBI-containing regimen and capped the thiotepa dose at 14 mg/kg (for patients given myeloablative regimen divided to 2 doses on Day -5 and -4) or at a dose of 10-12 mg/kg (in patients given a reduced intensity regimen). In addition patients were given BCNU at a dose of 400 mg/m^2 on Day -6 and fludarabine at a dose of 25 mg/m^2 on Day -6 to -4. We omitted entirely cranial irradiation, however Intrathecal (IT) therapy was allowed pretransplant (only) as per physician discretion. GVHD prophylaxis consisted of cycslosporine-methotrexate and ATG in cases of non-sibling donors. Results: Between July 2014 and June 2019, 22 patients from 3 centers in Israel were eligible for the protocol. Median follow up was 17 (range, 3-61) months. Median age at transplant was 51 (range, 22-68), Table. Majority of the patients (59%) had a CR1 with MRD negative disease . Ten patients (45%) were given a myeloablative regimen (>14 mg/kg of thiotepa) and 12 patients (55%) were given a reduced intensity regimen (10-12 mg/kg of thiotepa), majority (7 patients, 32%) because of age>60 years. Eight patients (36%) had a sibling donor, 12 patients (55%) had an unrelated donor and 2 (9%) had a 1 antigen-mismatch unrelated donor. GVHD prophylaxis consisted for all patients cyclosporine and a short course of methotrexate.Three patients (14%) developed sinusoidal obstruction syndrome (1 patient, severe). 7 patients (32%) had microbiology documented infections and 16 patients (73%) had grade 3-4 mucositis. All patients who survived (n=18, 82%) had a day 30-whole marrow donor chimerism > 96%. Cumulative incidences of grade 2-4 and grade 3-4 acute GVHD at day 200 were 51%(95% CI 35%-62%) and 11%(95% CI 5%-24%), respectively. Cumulative incidence of 2 year-moderate-severe chronic GVHD was 52% (95% CI 41%-74%). Relapse occurred in only 3 patients and all occurred within 4 months from HCT with a 2 year cumulative incidence of 17%(95% CI 12%-21%). Cumulative incidences of non-relapse mortality at 30 days, 3 months and 2 years after HCT were 15%(95% CI 8%-17%), 24%(95% CI 12%-32%) and 24%(95% CI 12%-32%), respectively. At time of data extraction 14 patients were alive. Cumulative Incidences of 2-year overall survival for CR1 patients with MRD negative disease and for non-CR1 patients with MRD negative disease at HCT were 70%(95% CI 61%-87%) and 62%(95% CI 50%-73%), respectively (p=.59), Figure. Factors associated in cox regression model with decreased overall survival were CNS involvement at diagnosis (p=.06, HR=2.24) and older age (p=.045, HR=1.5), while disease status at HCT, having a sibling donor, and intensity of conditioning were not predictive. Conclusions: This study shows that a thiotepa based regimen has substantial activity in patients with ALL, even in those who are older than 60 years and in patients intelligible to TBI. Reduced doses of thiotepa may have comparable efficacy and a lower toxicity profile when compared to higher doses and should be further investigated in this cohort of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal