BACKGROUND
Plerixafor (PLX) blocks the binding of stromal cell-derived factor to CXCR4, resulting in hematopoietic stem cell (HSC) release from the bone marrow. It has been successfully used as a mobilizing agent in poor mobilizers (PM). The best strategy is probably the "pre-emptive" (on demand) use, as it allows an "on time" identification of PMs, preventing collection failure and need for further mobilization. Usually a cut-off of < 10/ μL CD34+ cell count at leukocyte recovery is used for PLXa dministration, while a minimum level is not defined, as mobilization may be obtained even with very low values.
An important issue that has not been extensively explored so far is represented by the biological characteristics of the yields collected with PLX, especially clonogenicity, and its clinical counterpart, represented by the engraftment times observed after transplant.
In this report we retrospectively analysed our data on the use of PLX in PMs, evaluated its efficacy and focused on the clonogenicity of collected stem cells and engraftment post auto transplant (ASCT).
METHODS
We collected data on PM patients mobilized with PLX between 2011 and 2019:
Clinical data: mobilization and collection performance, transplant rate and engraftment times (days to PMN > 500/uL).
Biological data: Clonogenicity of HSC collected after PLX, measured by hematopoietic progenitor semi-solid cultures according to Stem Cell Technology.
These clinical and biological data were then compared to those obtained in patients mobilized without PLX and in allogeneic donors who received G-CSF in the same period.
RESULTS
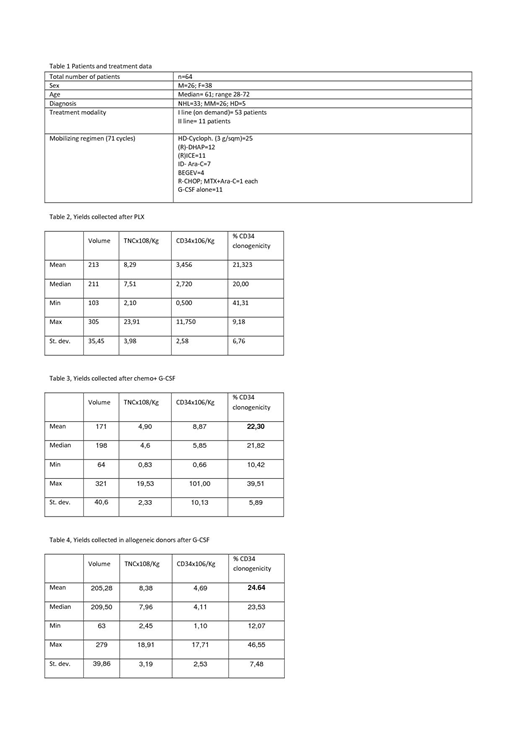
From January 2011 to June 2019 PLX was used in 73 mobilization cycles, performed in 64 patients. Patients characteristics are described in Table 1.
105 doses of PLX were given during 73 mobilization cycles (1,44 doses/cycle) and 93 collections performed (1,27/ cycle). Circulating CD34+ pre PLX administration were in median 6,81/µl (range: 0,27-21), while after treatment 26,20/µl (range: 4,8-155,4). Using a cut-off of ≥ 10/ μL CD34+, a successful mobilization was achieved in 67/73 cycles (success rate 91,7%). Overall 59 out of 64 patients achieved the collection target (92,2%) at any time and 54/64 have been transplanted (84,4%), the other 10 not yet due to mobilization failure (n=2), insufficient yield (n=3), clinical unfitness (n=2) or because too early (n=3).
A median of 4 HPC bags were reinfused (range: 2-12) and median time to WBC engraftment was 10 days (8-21). Engrafment times are in line with those of MM and NHL patients mobilized without PLX in the same period of time (median: 10, range: 8-12) at our Institution. Interestingly, 33 out of 35 pts mobilized with PLX (94,2%), compared to 442/449 (98,4%) engrafted in ≤11 days.
We analyzed the cellular composition of yields and clonogenicity of HSC collected in 3 different groups:
Group A: PLX mobilization (n=93)
Group B: chemo + G-CSF without PLX (n=755)
Group C: G-CSF mobilization in allogeneic donors (n=206)
We failed to demonstrate a statistical difference between clonogenicity in group A and B (p=0,691), while clonogenicity was slightly but significantly higher in group C compared to group A and B (p< 0,001).
Data are shown in tables 2,3 and 4.
CONCLUSION
In our cohort of patients, administration of PLX in PM resulted in successful mobilization of HPCs with good clonogenicity and engraftment potential. The use of PLX allowed an high proportion of patients to undergo ASCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal