Purpose: To study the efficacy of anti-CD19-CAR T-cell therapy and the changes of donor chimerism in CAR-T cells in vitro culture for patients who relapsed after allo-HSCT with a low level of donor chimerism.
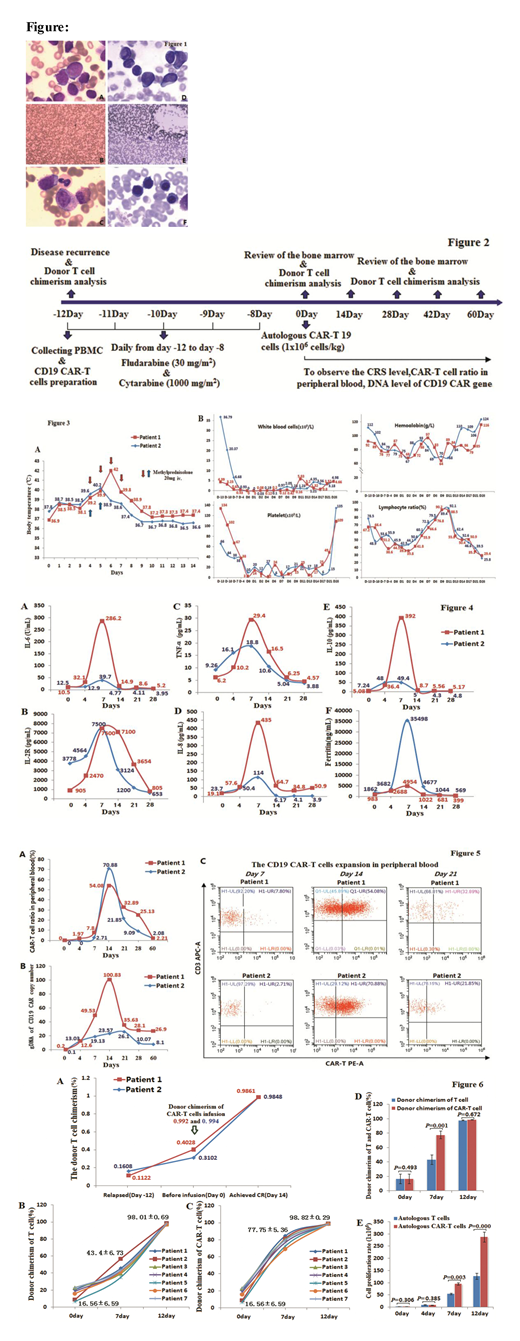
Methods: Two patients relapsed after allo-HSCT with a low level of donor chimerism (11.22% and 16.08%). Their autologous PBMCs were selected for anti-CD19-CAR T-cell therapy because their sibling or unrelated donor could not provide PBMC cells. The adverse events (AEs) were observed after CAR T-cell infusion. The cytokine release syndrome (CRS), the expansion of anti-CD19-CAR T-cells, the DNA level of anti-CD19-CAR gene and the changes of the donor chimerism were detected after CAR T-cell infusion. Then we selected other 7 acute leukemia patients relapsed after allo-HSCT with a low level of donor chimerism to detect the changes of donor chimerism in their T cells and anti-CD19-CAR T-cells in vitro culture.
Results: Their notable AEs were grade 2 CRS. They had no aGVHD during anti-CD19-CAR T-cell therapy. They achieved CR 14 days after anti-CD19-CAR T-cell therapy with a restoration of complete donor chimerism (98.61% and 98.48%). To date, they had leukemia-free survival (LFS) and complete donor chimerism for 8 and 7 months. It was very interesting and fortunate that the CAR-T cells infused into the two patients were restorated to complete donor chimerism (99.2% and 99.4%). Then we found that complete donor chimerism were restored after 12 days culture in vitro in all the T cells and anti-CD19-CAR T-cells in the other 7 patients relapsed after allo-HSCT with a low level of donor chimerism.
Conclusions: Anti-CD19-CAR T-cells derived from patients relapsed after allo-HSCT with a low level of donor chimerism were effective for this salvage therapy and could restorate to complete donor chimerism after 12 days culture in vitro.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal