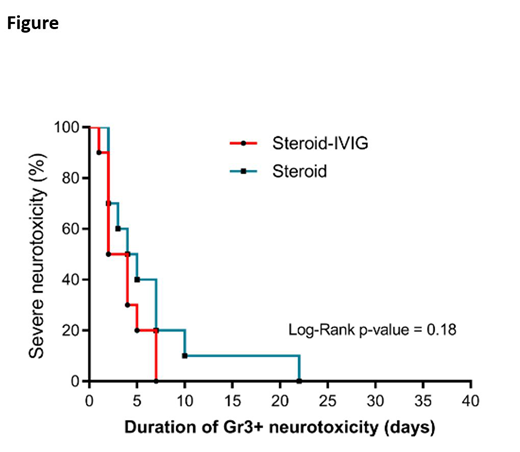
INTRODUCTION: Severe neurotoxicity occurs in ~30% of Diffuse Large B Cell Lymphoma (DLBCL) patients treated with CAR-T cell therapy. The current treatment for severe neurotoxicity is glucocorticoids +/- tocilizumab (an IL-6 antagonist) depending on concurrent cytokine release syndrome. Even with these treatments, neurotoxicity can have a complicated course. It is therefore essential to find the optimal treatment to reverse neurotoxicity timely. METHOD:This is a retrospective cohort study of neurologic and oncologic outcomes among patients with grade ≥ 3 neurotoxicity treated with glucocorticoids and IVIG compared to glucocorticoids only. Severe neurotoxicity was defined as grade 3 and graded by CRES/CARTOX score. Time to resolution of severe neurotoxicity (TTR) was defined as improvement of severe neurotoxicity to grade ≤ 2. RESULTS: We identified a total of 20 patients who received CAR-T therapy and developed severe neurotoxicity. Ten patients received glucocorticoids and IVIG (group A) and ten patients received glucocorticoids alone (group B). The median age was 62 (range: 52-74) for group A vs 64 years (range: 48-75) for group B. Both groups had similar ECOG performance status (p=0.17), IPI scores (p=0.34), and onset of severe neurotoxicity (median=6 days in both groups). Median TTR was 3 days (range, 1-7) for group A and 4.5 days (range, 2-22) for group B. There was no significant difference in TTR of severe neurotoxicity among both groups (Log-rank p=0.18, Figure). The median time to administration of IVIG after initiation of glucocorticoids was 2 days (range, 0.5-8). The median TTR following initiation of IVIG was 0.5 day (0.5-4). The objective response rate at 30 days was 80% in both groups. None of the patients who received IVIG developed thromboembolism, renal failure, autoimmune hemolytic anemia or acute lung injury. CONCLUSIONS: Although the use of IVIG during severe neurotoxicity after CAR-T therapy appeared to be safe, this pilot retrospective analysis demonstrated no significant difference in resolution of severe neurotoxicity with addition of IVIG to glucocorticoids. Further controlled studies limiting selection bias inherent in this retrospective analysis will help to determine the efficacy of IVIG in severe neurotoxicity in the context of CAR-T cell therapy.
Mokhtari:KITE PHARMA: Other: Clinical Advisor; NOVARTIS: Other: Clinical Advisor. Bachmeier:Kite/Gilead: Speakers Bureau. Jain:Kite/Gilead: Consultancy. Forsyth:Department of Defense: Research Funding; Pfizer: Research Funding; State of Florida Bankhead Coley: Research Funding; Moffitt Center of Excellence Celgene Project: Research Funding; Florida Academic Cancer Center Alliance: Research Funding; NIH/NCI 1R21 Grant: Research Funding; NIT DT Study Section Grant Review: Membership on an entity's Board of Directors or advisory committees; AbbVie Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ziopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tocagen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BTG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Inovio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novocure: Consultancy, Membership on an entity's Board of Directors or advisory committees. Locke:Novartis: Other: Scientific Advisor; Cellular BioMedicine Group Inc.: Consultancy; Kite: Other: Scientific Advisor. Lazaryan:Kadmon: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal