J Li, L Bao, ZJ Xia and KY Ding contributed equally to this study.
Background: Based on the promising results shown in the phase 3 trial (TOURMALINE-MM1, NCT01564537) and the China Continuation Study of MM1, the oral proteasome inhibitor (PI) ixazomib (ixa) was approved in China in April of 2018, in combination with lenalidomide (len) and dexamethasone (dex) (IRd), for patients (pts) with relapsed/refractory multiple myeloma (RRMM). Data on the efficacy and safety of ixa-based therapy in Chinese pts with MM in real-life practice is rather limited. A large national, multi-center, real-world study involving 14 centers from different areas of China was performed to investigate the current status of ixa usage in China and to evaluate the efficacy and safety in routine clinical practice. A total of 246 ixa-treated MM pts was enrolled, with 163 (66.3%) RRMM, 60 (24.4%) newly diagnosed MM and 23 (9.4%) pts received ixa as maintenance. Herein, we reported the data of RRMM in this study.
Methods: Medical records, including demographics, disease characteristics, treatment regimen and duration, response rate, adverse events (AEs) and survival, of ixa-treated (at least one cycle completed with response evaluation result) RRMM pts were analyzed.
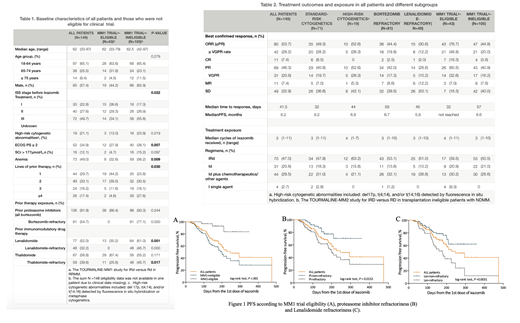
Results: A total of 149 evaluable pts (out of 163 RRMM pts) treated from April 2018, to July 2019 were included in analysis. Baseline features and prior treatment are summarized in Table 1. Patients were categorized into MM1 trial-eligible/-ineligible groups according to the inclusion and exclusion criteria of MM1 study. Median age was 62 years (range 33 - 87) with 52 (34.9%) ≥65 years. Most pts (75.2%) had ISS stage II-III disease. High-risk cytogenetic abnormalities (including del 17p, t (4;14), and/or t (14;16)) were detected in 19 patients (21.1%, among 90 patients with FISH results). Fifty-two (34.9%) pts had a ECOG PS ≥2. Overall, ixa-based regimens were used as the 2nd/3rd/4th/≥5th-line therapy in 29.7%, 33.1%, 16.2% and 17.4% of the pts, respectively. Prior treatment included bortezomib (91.9%), len (52.0%) and thalidomide (58.8%). More than half pts (54.7%) were refractory to previous bortezomib treatment, and 32.2% pts were len-refractory. MM-1 trial-ineligible pts had more advanced ISS stage, higher ECOG PS, more severe anemia, more lines of prior therapy and more refractory diseases.
Treatment, outcome and survival were listed in Table 2. Ixa-based regimens included IRd in 70 (47.0%) patients, ixa-dex (Id) in 31 (20.8%) patients and Id plus chemotherapeutics/other agents (44, 29.5%; including cyclophosphamide in 14 pts, thalidomide in 12 pts, adriamycin in 6 pts, melphalan in 5 pts and daratumumab in 3 pts) in 20 (33.3%). (Table 2). One patient received stem cell transplantation (SCT) during follow-up.
The best confirmed ORR (≥PR) for all 149 patients was 53.7% (80/149), including 28.2% of patients with ≥VGPR and 7.4% with a CR, with a median time to response of 41.5 days. Surprisingly, ixa-based regimens demonstrated efficacy in pts with PI/len refractory diseases, with an ORR and ≥VGPR rate of 44.4% and 19.9% for PI-refractory pts, and an ORR and ≥VGPR rate of 30.6% and 12.2% for len-refractory pts. Pts eligible for MM1 study shown comparable ORR (76.7%) with that reported in MM1 (ORR 78%). No significant difference in response between different ixa-based regimens was observed.
The median PFS of the whole cohort, pts with standard/high cytogenetic risks, pts refractory to bortezomib/len and pts eligible/ineligible for MM1 trial was 8.2, 8.2, 6.8, 6.7, 5.9months, not reached and 6.6months respectively. The median overall survival (OS) of the whole cohort and every subgroup was not reached.
Adverse events (AEs) of grade 3/4, reported in 40 (27.2%) patients, included 10.1% thrombocytopenia, 5.4% anemia, 3.4% diarrhea and 6.0% pneumonia. Only 3 (2.01%) pts had a grade 3/4 peripheral neuropathy during follow-up.
Discussion and conclusion:
Our results show that ixa-based therapy demonstrated good efficacy with limited toxicity for pts with RRMM in real-life clinical practice. Moreover, in pts with PIs- or len- refractory diseases, ixa-based therapy still showed acceptable effectiveness (ORR: 44.4% and 30.6%; mPFS: 6.7 months and 5.9 months). Although 70.5% pts in our real-life cohort were ineligible for MM1 trial, the efficacy and safety profile is similar to that reported in MM1 China Continuation Study. Ixa-based therapy is a reasonable choice for Chinese RRMM pts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal