Introduction: Vitamin D deficiency is common, with 25-50% of the population having levels below the optimal range (<20 ng/mL). Aside from its role in maintaining serum calcium and skeletal homeostasis, vitamin D has been shown to play an important role in regulation of differentiation, proliferation, apoptosis, metastatic potential and angiogenesis in a variety of malignancies. Low serum 25(OH)D levels have been associated with a worse prognosis in several malignancies, including colorectal and breast cancer, chronic lymphocytic leukemia and non-Hodgkin's lymphoma.
Almost no data have been reported on vitamin D levels in light chain (AL) amyloidosis. We therefore measured vitamin D levels among AL patients and correlated levels at presentation to explore if vitamin D deficiency has any effect or association in this disease.
Methods: Stored serum samples from 90 patients with newly diagnosed AL amyloidosis were obtained for vitamin D studies, which included 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D [1,25(OH)2D] and vitamin D binding protein (DBP). All vitamin D measurements were made by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Total 25(OH)D and 1,25(OH)2D was assessed as the additive sum of the 25(OH)D2/25(OH)D3 and 1,25(OH)2D2/1,25(OH)2D3 components, respectively. Clinical data was extracted from the electronic medical records. All calculations were made using JMP software (SAS, Cary, NC).
Results: All patients were seen between June 2004 and September 2013. Median age was 63 [IQR 57-70]. Heart, kidney, nerve, liver and gastrointestinal amyloidosis was seen in 80%, 60%, 19%, 17% and 13% of patients, respectively. The median dFLC and bone marrow plasma cells were 28 (IQR 12-84) mg/dL and 10% (IQR 5-20%), respectively.
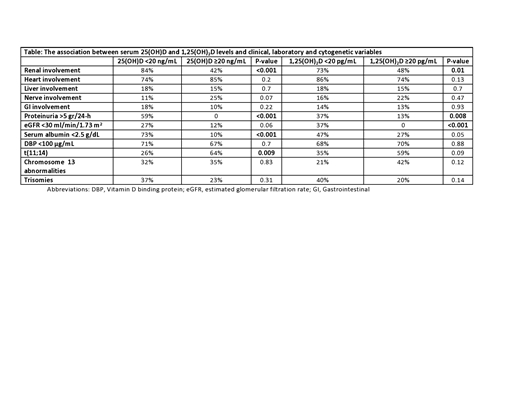
The median serum 25(OH)D level was 20 ng/mL (IQR 10-29; normal >20 ng/mL), while the median serum level of 1,25(OH)2D was 23 pg/mL (IQR 14-37; normal 16-64 pg/mL for men; 16-78 pg/mL for women). 25(OH)D levels <20 ng/mL were strongly associated with renal involvement (84% renal involvement vs 42% of renal involvement for levels ≥20 ng/dL, P<0.001; Table). No other association with organ involvement was seen. Among all patients, heavy proteinuria (>5 gr/24-h) was strongly associated with low 25(OH)D, (P<0.001, Table). A weaker association was seen between 25(OH)D levels and estimated glomerular filtration rate (eGFR) (P=0.06). Seventy-eight percent of patients with 1,25(OH)2D levels below 20 pg/mL had renal involvement versus 48% in those with levels above 20 pg/mL (P=0.01). While heavy proteinuria was associated with 1,25(OH)2D (P=0.008), the association was stronger for eGFR, as no patient with an eGFR <30 ml/min/1.73 m2 had a 1,25(OH)2D level above 20 pg/mL (P<0.001). An association between vitamin D levels and low serum albumin was also seen, mainly in relation to 25(OH)D (Table). The median level of serum DBP was 58 μg/mL (IQR 36 - 120 μg/mL), significantly lower than the normal range (168-367 μg/mL). Low levels of 25(OH)D or 1,25(OH)2D were not associated with lower DBP levels. Surprisingly, patients with renal involvement had higher levels of DBP versus those without renal involvement (median 70.9 vs 48 μg/mL; P=0.03), although median levels were below normal in both groups.
FISH data was available in 47 of patients (52%). Patients with 25(OH)D levels <20 ng/dL were less likely to harbor t(11;14) versus patients with 25(OH)D levels above this threshold (26% vs 64%, respectively; P=0.0095). A similar, but less significant association was seen between 1,25(OH)2D levels at a 20 pg/mL threshold and t(11;14) (35% vs 59%, P=0.09). Other associations between vitamin D and FISH abnormalities were not seen.
Conclusions: Hypovitaminosis D is common among AL amyloidosis patients with renal involvement. In the general cohort, heavy proteinuria, reduced eGFR and low serum albumin were all associated with low circulating vitamin D levels. Although the majority of AL patients had low levels of DBP, urinary loss of DBP does not explain the low levels of vitamin D. Hypovitaminosis D is associated with non-t(11;14) disease, a finding which warrants further exploration.
Dispenzieri:Akcea: Consultancy; Intellia: Consultancy; Janssen: Consultancy; Pfizer: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Alnylam: Research Funding. Lacy:Celgene: Research Funding. Dingli:Rigel: Consultancy; Karyopharm: Research Funding; Millenium: Consultancy; Janssen: Consultancy; alexion: Consultancy. Kapoor:Cellectar: Consultancy; Celgene: Honoraria; Sanofi: Consultancy, Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; Janssen: Research Funding; Glaxo Smith Kline: Research Funding. Leung:Omeros: Research Funding; Aduro: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding. Russell:Imanis: Equity Ownership. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Gertz:Ionis/Akcea: Consultancy; Alnylam: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Annexon: Consultancy; Prothena Biosciences Inc: Consultancy; Spectrum: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal