Background: The treatment of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) has continued to evolve in recent years, offering the patients different therapeutic options. Venetoclax (ven) is a selective, small molecule inhibitor of B-cell receptor-2 (BCL-2) approved by the FDA for patients with newly diagnosed and relapsed/refractory CLL. Stratification based on tumor lysis syndrome (TLS) risk is recommended and may guide debulking strategies.
Methods: We retrospectively analyzed 36 patients with relapsed/refractory (RR) CLL who received treatment with ven at the Moffitt Cancer Center between January 2016 and July 2019. Objective response to therapy was determined based on iwCLL. Progression free survival (PFS) and overall survival (OS) were evaluated via Kaplan-Meier method; overall response rate (ORR) and complete response (CR) via Fisher's exact test. Adverse events (AEs) were graded by CTCAEv5.
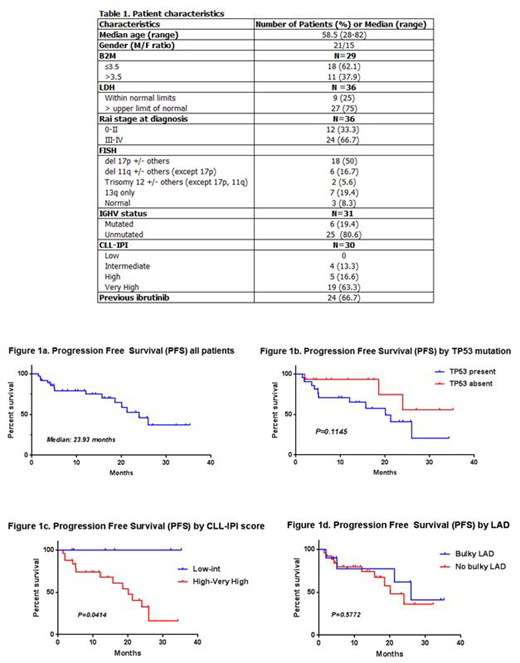
Results: The median age was 58.5 years (28-82). Median follow up was 12.97 months. The vast majority of patients had high risk disease; Chromosomal analysis by Fluorescence In Situ Hybridization (FISH) reported Del17p and Del11q (+/- others, except Del17p) in 18 (50%) patients and 6 (16.7%) patients respectively. Twenty one (58.3%) patients had a TP53 mutation by next generation sequencing. Twenty five (80.6%) patients had unmutated IGHV status, and 24 (79.9%) patients had high or very high CLL-International prognostic Index (CLL-IPI) score. Twenty-four patients (66.7%) had been previously treated with Ibrutinib, 11 of those progressing on it. Further characteristics are described in table 1.
Median OS was not reached. Median PFS was 23.93 months, figure 1a. Median duration of response was 25.97 months. The ORR (PR+CR) at the time of analysis was 63.9%, with CR rate of 33.3%, and PR rate of 30.6%. Nine patients (25%) had stable disease (SD), while four patients (11.1%) progressed on treatment. Minimal residual disease (MRD) was evaluated as part of the response assessment in 7 patients (19.4%) by flow cytometry (1 patient) and by clonoSEQ (6 patients), and 4 (11.1%) had achieved undetectable disease. Response rates were also based on risk stratification; OS and PFS were not affected by the presence of TP53 mutation (p=0.1145) as described in figure 1b, IGHV unmutated status, or NOTCH1 mutation. PFS was inferior in patients with high-very high CLL-IPI score (p=0.0414), figure 1c. The presence of bulky lymphadenopathy (≥5 cm) did not affect outcomes (p=0.5772), figure 1d.
Reasons for treatment discontinuation were: progressive disease (PD) in 6 (16.7%), MD/patient preference in 5 (13.9%), Richter's transformation in 2 (5.6%), AEs in 1 (2.8%), allogeneic transplant in 1 (2.8%), and death while on treatment in 1 (2.8%) case.
Twenty patients (55.6%) were considered to be at intermediate/high risk of developing TLS, of whom 15 (75%) received debulking therapy with rituximab (1), ofatumumab (11), or obinutuzumab (3) prior to ven. Based on the absolute lymphocyte count (ALC), the risk of TLS became low in 12 patients. The use of a debulking monoclonal antibody (MoAb) improved lymphocytosis, but did not impact PFS.
Treatment was well tolerated, and was not dependent on the use of debulking MoAb; however, out of the 18 patients (50%) who underwent debulking therapy, 9 (25%) had grade 3/4 toxicities. Neutropenia was the most common grade 3/4 toxicity in all patients, which was seen in 7 (19.4%) patients, and diarrhea was the most common grade 3/4 non-hematologic toxicity, seen in 4 (11.1%) patients; grade 3/4 neutropenia was seen in 4 (57.1%) of those patients who received debulking therapy, while diarrhea was only seen in 1 (25%). No cases of TLS were observed. Ten patients (27.8%) were admitted for ramp up of ven as per physician preference, independent of TLS risk and comorbidities.
Conclusion: Venetoclax is a very active agent for R/R CLL with an acceptable toxicity profile. Debulking strategy is a tolerable option for patients with high burden disease and may reduce the incidence of TLS and/or hospitalization. Nonetheless, based on this retrospective study, it does not seem to have a significant impact in outcomes, and it carries a higher risk for potential cumulative toxicity.
Chavez:Genentech: Speakers Bureau; Kite Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals, Inc.: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Sandoval-Sus:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Shah:Celgene/Juno: Honoraria; Kite/Gilead: Honoraria; Incyte: Research Funding; Jazz Pharmaceuticals: Research Funding; Pharmacyclics: Honoraria; Adaptive Biotechnologies: Honoraria; Spectrum/Astrotech: Honoraria; Novartis: Honoraria; AstraZeneca: Honoraria. Bello:Celgene: Speakers Bureau. Sokol:EUSA: Consultancy. Nodzon:Pfizer: Consultancy; Pharmacyclics: Consultancy; Genentech: Consultancy, Other: Speaker Fees; Abbvie: Other: Speaker Fees. Pinilla Ibarz:Bayer: Speakers Bureau; TG Therapeutics: Consultancy; Teva: Consultancy; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Sanofi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal