Background
Myelodysplastic syndrome (MDS) is a clonal, pre-leukemic stem cell disorder characterized by decreased blood counts due to ineffective hematopoiesis. Plasmacytoid dendritic cells (PDC) are stem cell derived, type I interferon producing dendritic cells, that are readily identifiable by flow cytometry (FC) using expression of CD123 and HLA-DR. PDCs have been shown to be decreased in acute myeloid leukemia (AML). Our study uses FC to evaluate PDC in MDS, their relationship to the Revised International Prognostic Scoring System (IPSS-R) for MDS, as well as their relationship to clinical outcomes.
Methods
We identified 197 patients with new MDS diagnoses and examined FC data of bone marrow aspirates (BMA) at first presentation to our institution for blast and PDC percentages. Patients who presented with an outside diagnosis of MDS greater than 1 year before presentation to our institution were excluded. MDS with excess blasts 1 and 2 (MDS-EB1/EB2) were designated as high grade, while MDS with isolated del5q (MDS-d5q), single lineage dysplasia (MDS-SLD), and multilineage dysplasia (MDS-MLD) were designated as low grade, with or without ring sideroblasts. 163 patients had sufficient data to calculate their IPSS-R risk categories. Sixteen patients with a history of solid tumor malignancies undergoing BMA for cytopenias were used as controls. The bone marrow of these control patients showed no evidence of morphologic dysplasia, they had normal karyotypes, and no pathogenic variants were detected on a 28 gene next generation sequencing based myeloid panel. We used CD34 and CD117 to quantify blasts, and CD123 and HLA-DR to quantify PDCs.
Results
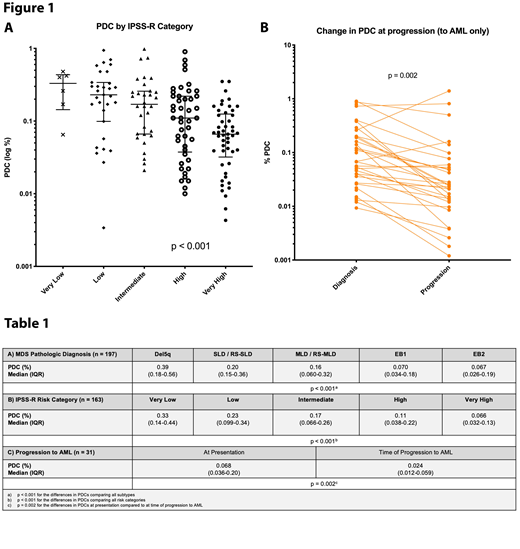
The proportion of PDCs expressed as a percentage of total WBCs was significantly lower in higher risk MDS diagnoses (p <0.001) (Table 1A), as well as with worsening IPSS-R risk category (p <0.001) (Table 1B, Figure 1A). Sixteen percent of patients received disease modifying therapy (hypomethylating agents or lenalidomide) prior to presentation at our institution; PDC proportions in these patients were not significantly different from patients who presented untreated (p = 0.79). In the entire cohort, a lower proportion of PDC at the time of presentation was significantly associated with an increased risk of death (one-unit decrease in log PDC, HR 2.42, 95% CI 1.01-5.78, p = 0.046), but was not significantly associated with risk of progression to AML. Thirty-one patients progressed to AML, and these patients showed a significant decrease in the proportion of bone marrow PDCs at the time of progression to AML (p=0.002) (Table 1C, Figure 1B).
Discussion
This study demonstrates that PDC proportions decrease with MDS disease progression and are progressively lower as IPSS-R risk category increases. We also demonstrate that quantification of PDC in MDS can aid in predicting outcomes, although this may be due to a strong association with IPSS-R categories. FC is a useful and clinically feasible tool for quantitating PDCs in bone marrow aspirates, and this measurement is correlated with risk in MDS.
Arcila:Invivoscribe, Inc.: Consultancy, Honoraria. Roshal:Celgene: Other: Provision of Services; Auron Therapeutics: Equity Ownership, Other: Provision of services; Physicians' Education Resource: Other: Provision of services.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal