Background: More than 50% of patients with myelodysplastic syndrome (MDS) become transfusion dependent (TD) during the course of their disease and 25-30% present as TD at diagnosis. While TD is more common in IPSS/IPSS-R higher risk patients and is associated with inferior overall survival, it is unclear if achievement of transfusion independence (TI) for even short periods of time is associated with improved overall survival (OS).
Objectives: Evaluate the impact of intermittent transfusion dependence and independence on OS in MDS patients and compare the OS with patients persistently TD or TI.
Determine the optimal TI duration or ratio that translates into improved overall survival and the impact of developing transfusion dependence or acquiring transfusion independence after diagnosis.
Methods: We extracted the detailed clinical and transfusion records of patients followed since 2010 in the national MDS registry of Canada (MDS-CAN) and assigned patients into 4 categories: TI continuous (TIcont), TD continuous (TDcont), TI followed by TD (TI/TD) and TD followed by TI (TD/TI) at any time. TD was defined as receiving at least 1 unit of packed red blood cells (PRBC) within an 8-week period for a consecutive 16 weeks. The ratio of time spent TI to total follow up period was calculated for each patient. Survival was compared between groups and an ROC curve was attempted to define the optimal ratio of TI/follow-up that translated into an overall survival benefit.
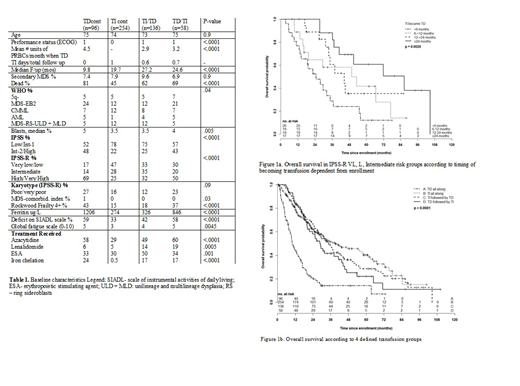
Results: This study evaluated 544 patients with a median follow up of 19 months (95% CI 18-22 and actuarial OS of 28 months (95% CI 24-31). 254 (46%) were TIcont, 96 (18%) TDcont, 136 (25%) TI/TD (median time to TD 7.4 months, interquartile range (IQR) 4-19) and 58 (11%) TD/TI (median time to TI 6 months (IQR 4-10) lasting a cumulative13 months (IQR 7-29). Baseline characteristics comparing these groups are in table 1. Patients TDcont and TD/TI had higher risk IPSS/IPSS-R scores, more unfavourable karyotypes, a greater degree of frailty, higher ferritin and levels of fatigue and more deficits in instrumental activities of daily living at enrollment. 57% of TI/TD patients remained TD while 43 % converted back to TI for variable lengths of time. Among the TD/TI patients, 46% remained transfusion independent (median TI duration 12 mos, IQR 6-21) while 53% converted back to TD (median TI duration 12 mos, IQR 6-29). The TI ratio was 0.7 +/-0.4 overall. The receiver operating curve could not identify a threshold ratio or duration of transfusion independence that predicted with good sensitivity and specificity overall survival. In the 304 patients with IPSS-R very low, low and intermediate risk scores, 168 (55%) were TIcont, 27 (9%) TDcont, 81(27%) were TI/TD and 28 (9%) were TD/TI. In lower risk TI/TD patients, the development of TD within the first 6, 6-12, 12-24 and >24 mos from enrollment was associated with progressively worse OS (25, 50, 45 and 86 mos respectively, p=.0025) (figure 1a). In the 58 TD/TI patients, OS did not differ if the achievement of TI occurred < 6, 6-12 or >12 mos from enrollment (p=0.3). Of all 48 TD/TI patients with an overall survival that exceeded 12 months, there were no significant differences in OS if the duration of transfusion independence lasted a minimum of 16, 24 or 48 weeks (p=0.45). The actuarial OS for the 4 transfusion categories were 39 months (TIcont), 35 months (TI/TD), 29 months (TD/TI) and 10 months (TDcont), p<.0001(figure 1b).
Conclusion: This study validates the extremely poor prognosis associated with persistent transfusion dependence. While only 38% of patients TD at diagnosis achieve transfusion independence at any point, the achievement of transfusion independence (even if intermittent) is associated with an improved overall survival that is similar to TIcont and TI/TD within the first 2 years of follow up. For patients with TD who achieved TI, we were unable to determine a TI duration or ratio that translated into a survival benefit. While the achievement of transfusion independence may simply reflect response to therapy or better disease biology, our data suggest that we should strive for the acquisition and/or maintenance of transfusion independence as it may be a surrogate for improved overall survival. The impact of achieving or losing TI on health related quality of life is being analyzed.
Buckstein:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding. Geddes:Alexion: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Sabloff:Pfizer Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ASTX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi Canada: Research Funding; Actinium Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Keating:Novartis: Honoraria; Seattle Genetics: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Hoffman La Roche: Membership on an entity's Board of Directors or advisory committees. Leber:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Leitch:Alexion: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau; Otsuka: Honoraria; AbbVie: Research Funding; Celgene Corporation: Honoraria, Research Funding. Yee:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffman La Roche: Research Funding; MedImmune: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Millennium: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Research Funding. St-Hilaire:Amgen: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Finn:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Ipsen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Boehringer Ingelheim: Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nevill:Paladin Labs: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Storring:Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees. Shamy:Amgen: Membership on an entity's Board of Directors or advisory committees; Abbie: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding. Banerji:LLSC: Research Funding; Research Manitoba: Research Funding; CCMF: Research Funding; Abbvie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Honoraria; CIHR: Research Funding; CancerCare Manitoba/University of Manitoba: Employment; CAPhO: Honoraria; BIOGEN: Other: Licensing fee; Dana-Farber Cancer Institute: Other: Licencing fee; Janssen: Consultancy, Honoraria, Research Funding; Roche: Honoraria, Licensing fee, Research Funding. Delage:Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Wells:Novartis: Honoraria, Research Funding; Alexion: Honoraria, Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal