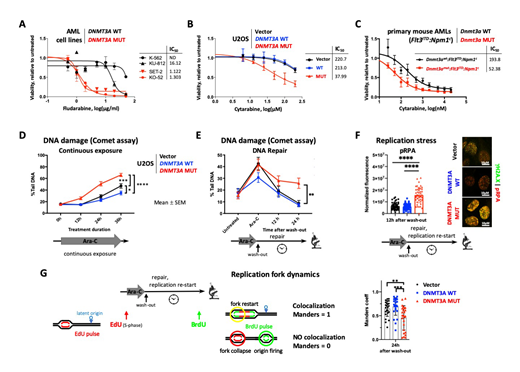
Mutations in the DNA methyltransferase 3A (DNMT3A) gene are recurrent in de novoacute myeloid leukemia (AML) and are associated with poor prognosis. Although studies demonstrated survival benefit of induction chemotherapy dose intensification, outcomes remain unsatisfactory in most patients due to advanced age, comorbidities, and hence inability to tolerate treatment. Clinical trials of low-intensity regimens combining cytarabine and cladribine, nucleoside analog chain terminators that stall DNA replication, appear to be safe and effective, and tend to particularly benefit patients with DNMT3Amutations. Consistently, we observe increased sensitivity to cytarabine, fludarabine, and cladribine in multiple cellular systems harboring mutant DNMT3Ain vitro (Figure 1A, B). Differential sensitivity to cytarabine was confirmed in normal and leukemic primary bone marrow cells derived from mice with and without Dnmt3a mutations ex vivo (Figure 1C).
Dynamic chromatin organization plays a pivotal role in DNA-associated cellular processes including DNA replication and damage repair. We previously found altered chromatin remodeling in cells expressing mutant DNMT3A after genotoxic stress. Gene expression studies by us and others demonstrated negative enrichment of cell cycle related signatures including G2/M checkpoint adaptation, in cells with DNMT3A mutations. These signatures are implicated in DNA damage response and replication fork integrity and suggest sensitivity to replication stress. To investigate the mechanism of differential sensitivity to cytarabine-induced DNA damage, we overexpressed wildtype (WT) or R882 mutant (MUT) forms of DNMT3A in U2OS cells, a well-established model for DNA damage studies. Analysis of the DNA damage signaling proficiency in response to cytarabine revealed persistent intra-S phase checkpoint activation (phospho-CHK1), accompanied by accumulation of DNA damage visualized by γH2A.X foci and by Comet assay in the DNMT3A(mut) overexpression cells (Figure 1D). This damage was only partially resolved after drug had been removed and cells were allowed to repair the DNA (Figure 1E), and was carried through mitosis, resulting in increased rate of micronucleation.At the same time, DNMT3A mutant cells remained proficient in initiating homology-directed repair (HDR) and non-homologous end joining (NHEJ) pathways, evidenced by RAD51 and 53BP-1 foci formation, respectively. These data demonstrate enhanced sensitivity to cytarabine in cells expressing mutant DNMT3A is due to increased susceptibility to DNA damage during replication, rather than defects in double-strand DNA break repair. In support of this, cells with mutant DNMT3Awere characterized by accentuated replication stress as evidenced by high levels of phospho-RPA, which persisted after drug wash-out (Figure 1F). Consistently, DNMT3A-mutant cells treated with cytarabine were characterized by a higher number and a larger area of PCNA foci. Pulse-chase double-labeling experiments with EdU and BrdU after cytarabine wash-out demonstrated that while the overall kinetics of replication restart remained unchanged, cells with DNMT3A(mut) showed higher rate of fork collapse and increased reliance on latent replication origins (Figure 1G). Gene expression profiling by RNA-seq identified dysregulation of pathways associated with cell cycle progression, specifically G1/S phase transition, DNA replication, DNA integrity checkpoint, and chromatin.
Our studies show cells with DNMT3A mutations have a defect in recovery from replication fork arrest and subsequent accumulation of unresolved DNA damage, which may have therapeutic tractability. These results demonstrate, in addition to its role in epigenetic control, DNMT3A contributes to preserving genome integrity during DNA replication and suggest that cytarabine-induced replication fork stalling may further synergize with other agents aimed at DNA damage and replication.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal