Introduction:
Approximately 20% of patients with Hodgkin lymphoma (HL) are aged 60 years or older. There is no standard of care for such patients, who have a markedly inferior prognosis compared with younger patients and are significantly underrepresented in clinical trials. Escalated BEACOPP chemotherapy has been demonstrated to be highly effective in frontline HL management, but when used in older patients (even at non-escalated doses) there is an unacceptable rate of toxicity-related death. We proposed that a modification of the BEACOPP regime by removing bleomycin and etoposide and dose reduction of cyclophosphamide ('ACOPP') would potentially be a well-tolerated and effective regimen for older HL patients.
Methods:
This is a single centre pilot study investigating the feasibility of ACOPP chemotherapy for frontline management of older patients with HL who were deemed unfit for more intensive management approaches. Patients received intravenous (IV) Doxorubicin (35mg/m2) and Cyclophosphamide (650mg/m2) on day 1 along with oral Procarbazine (100mg/m2) for 7 days and oral Prednisolone (40mg/m2) for 14 days. IV Vincristine (1.4mg/m2, max 2mg) was given on day 8 and subcutaneous granulocyte colony stimulating factor given on days 9-13. Treatment was delivered as an outpatient every 21 days to a maximum of 6 cycles. Interim PET/CT assessment was performed after 2 cycles of treatment.
Results:
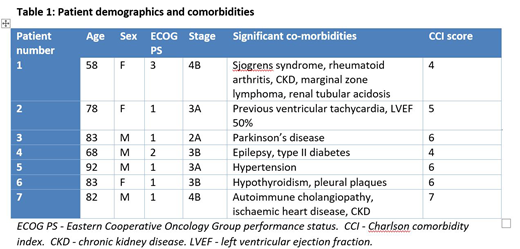
Seven patients with a median age of 81 years (range 58-92) were treated between March 2018 - March 2019. All patients had histologically confirmed classical Hodgkin lymphoma. 6/7 patients had advanced stage disease with median IPS 3. One patient had unfavourable stage 2 disease by EORTC criteria. Median Charlson Comorbidity Index (CCI) score was 6 with further details outlined in Table 1.
All 7 patients completed at least 2 cycles of therapy and all had a complete metabolic response on interim PET with ongoing response confirmed on end of treatment CT. Four patients completed 6 cycles of therapy, with the other 3 patients completing 2, 4 and 5 cycles respectively. Reasons for completing less than 6 cycles of therapy were physician concerns over increased frailty in patient 5, concurrent diagnosis of Parkinsons disease with resultant physical deconditioning in patient 3 and postural hypotension (possibly secondary to vincristine) in patient 7.
Grade 3/4 neutropenia was seen in 5/7 patients but only 1 patient had an episode of febrile neutropenia. Thrombocytopenia (2 patients, both grade 3, none requiring platelet transfusion) and anaemia (5 patients, all grade 3 and required red cell transfusion) were also seen. Peripheral neuropathy occurred in 2 patients (grade 1, grade 2). 6/7 patients required admission to hospital during therapy - primary reasons for admission were infection (n=2), constipation (n=2), anaemia (n=1) and autonomic neuropathy (n=1).
All 7 patients remain alive and in remission at time of analysis (median FU 9 months).
Conclusion:
We have demonstrated the feasibility of ACOPP as a chemotherapy regimen which can be delivered in an outpatient setting to elderly patients with HL. We would highlight the median age of 81 years and significant co-morbidity burden of the patients in this pilot study. All patients achieved a negative interim PET and remain in remission at the time of analysis. Toxicity was mainly haematological but was manageable with only 1 episode of febrile neutropenia and no platelet transfusion required. The majority had hospital admissions during therapy, with only 4/7 managing to complete six cycles of treatment. Definitive conclusions regarding efficacy and safety cannot be drawn from this small number of patients but the high rates of metabolic response are encouraging and we feel the regimen merits further, prospective assessment in a large clinical trial.
Travers:Celgene: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees. McKay:Janssen: Honoraria, Speakers Bureau; Epizyme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Leach:Abbvie: Honoraria; Janssen: Consultancy, Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal