Background: Immune checkpoints, including PD-1/PD-L1, play an important role in immunosuppression in various malignancies. Elevated levels of soluble programmed death-ligand1 (sPD-L1) are associated with worse prognosis in multiple myeloma and diffuse large B cell lymphoma. Herein, the purpose of this study is to investigate the relationships between plasma sPD-L1 levels and clinical response in peripheral T-cell lymphomas (PTCLs) patients.
Methods: Data from three cohorts, including 11 ALCL patients, 28 PTCL-NOS patients and 81 matched normal control tissues, were also obtained from the ONCOMINE database (https://www.oncomine.org) for PD-L1 gene expression array. The comparison dataset analysis of PD-L1 mRNA levels among diverse PTCL subtypes and matched normal control tissues was performed.
Other 37 PTCLs patients and 20 healthy volunteers were also enrolled in this study. Peripheral blood from patients was collected prior to systemic therapy. Plasma levels of sPD-L1 and IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA). PD-L1 expression in tissues was detected by immunohistochemistry (IHC). Clinical response for patients was evaluated.
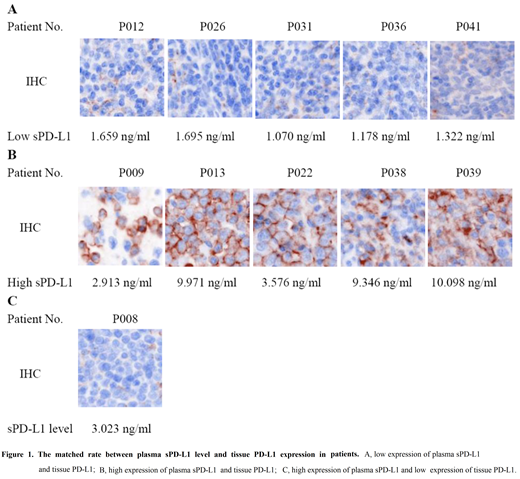
Results: ONCOMINE database analyses showed that the PD-L1 mRNA expression was upregulated in PTCLs, which were significantly higher in PTCLs compared to matched normal control tissues (1 cohort, P=0.029; 2 cohort, P=0.020 and 3 cohort, P=0.021). Among another cohort of 37 PTCLs patients and 20 healthy volunteers, the median sPD-L1 level was respectively 0.729 ng/ml for 20 healthy volunteers and 1.696 ng/ml for PTCLs patients, which was significantly higher than that in healthy volunteers (P=0.000). The median IFN-γ level of PTCLs patients was 4.555 pg/ml, and that the levels of sPD-L1 was positively correlated with the level of IFN-γ (P=0.000, r=0.849) for PTCLs patients. We found that patients with elevated LDH level, advanced stage and elevated β2-MG level had higher sPD-L1 levels than those with normal LDH level, early stage and normal β2-MG level. The sPD-L1 level was also positively correlated with LDH levels (P=0.003), clinical staging (P=0.045) and β2-MG level (P=0.045). Patients with high sPD-L1 level had lower overall response rate than those with low sPD-L1 level (88.9% vs 50.0%, P= 0.022), suggesting that sPD-L1 levels was an underlying plasma biomarker to predict the clinical response in PTCL patients. The median PFS for high and low sPD-L1 level groups was 42.7 months (95% CI, 27.9-57.6) and 53 months (95% CI, 33.7-72.3), respectively. As well, the median OS for high and low sPD-L1 level groups was 48.3 months (95% CI, 35.2-61.2) and 71 months (95% CI, 51.0-90.9), respectively. Survival data suggested that patients with high sPD-L1 levels tended to have shorter PFS and OS than those with low sPD-L1 levels. We also found that plasma sPD-L1 level appeared to have a positive relationship with tissue PD-L1 expression in PTCLs patients, and both of them had a high matched rate each other (90.9%).
Conclusions: PTCLs patients had higher sPD-L1 level compared with healthy volunteers. High sPD-L1 level was correlated with worse clinical response, suggesting that sPD-L1 level was an underlying plasma biomarker to predict the prognosis for PTCLs patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal