Introduction
Based on the cell of origin, diffuse large B-cell lymphoma (DLBCL) is divided into germinal center B-cell (GCB) and activated B-cell (ABC) like subtypes, which differ in their gene expression profiles and clinical presentation with the ABC DLBCLs showing a worse outcome in response to R-CHOP immunochemotherapy. However, composition of the tumor microenvironment (TME) of these molecular subtypes has not been characterized.
Methods
We used Hans algorithm to determine the molecular subtypes (GCB vs non-GCB) and multiplexed immunohistochemistry (mIHC) to characterize tumor infiltrating T-cell phenotypes, including cytotoxic T-cells (CTLs; CD8, Granzyme B, OX40, Ki67), T regulatory cells (Tregs; CD3, CD4, FoxP3), Th1 effector cells (CD3, CD4, TBET) and T-cell immune checkpoint (CD3, CD4, CD8, PD1, TIM3, LAG3) in 165 primary DLBCLs. The findings were correlated with the expression of human leukocyte antigens (HLA) I and II (beta-2 microglobulin (B2M), HLA-ABC and HLA-DR), and outcome of the patients treated with R-CHOP-like immunochemotherapy.
Results
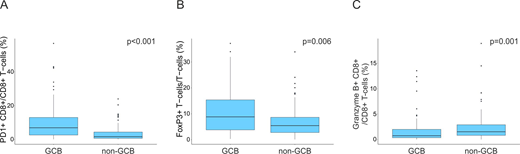
In the whole cohort, 82 (50%) cases were classified as GCB and 83 (50%) as non-GCB DLBCLs. In the GCB subtype, cytotoxic T-cells were more often PD1+, and T-cells FoxP3+than in the non-GCB subtype (Figure 1A-B). Furthermore, GCB DLBCLs tended to be more commonly HLA-DR+(p=0.102). In the non-GCB DLBCLs in turn, HLA I positivity was more frequent (B2M, P=0.007; HLA-ABC, p=0.108), cytotoxic T-cells more often granzyme B+(Figure 1C), T-cells TBET+(p=0.018) and LAG3+TIM3+(p=0.033). A high proportion of granzyme B+cells (p=0.002), PD1+cells (p=0.02) and TIM3+CD4+T-cells (p=0.006) from all cells translated to adverse overall survival (OS) in the patients with non-GCB DLBCL, all independent of the IPI. In contrast, a high proportion of TIM3+cells (p=0.015), and FOXP3+TBET+T-cells (p=0.005) from all cells were associated with poor OS in the patients with GCB DLBCL, also independent of the IPI.
Conclusions
TME differs significantly between GCB and non-GCB DLBCLs and has subtype-specific prognostic impact on survival.
Leppa:Roche: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal