Introduction: Acute Myeloid Leukemia (AML) is a biologically heterogeneous disease with poor outcomes despite aggressive induction chemotherapy (IC). Cytogenetics and mutational profile are used to inform subgroups of patients who are at high risk of relapse. Mutations in the fms-related tyrosine kinase 3 gene (FLT3) are detected in 30% of adults with newly diagnosed AML. The predominant (75%) FLT3 mutation, is an internal tandem duplication mutation (FLT3-ITD), which confers poor prognosis due to a high relapse rate. The addition of the multitargeted kinase inhibitor midostaurin to standard IC significantly prolonged overall survival (hazard ratio for death 0.77, p=0.016) among patients with AML and a FLT3 mutation. Midostaurin was approved by the United States Food and Drug Administration (FDA) in April 2017 for the treatment of adult patients with newly diagnosed FLT3+ AML, as detected by an FDA-approved test, in combination with standard cytarabine and daunorubicin IC and cytarabine consolidation. The recommended dose of midostaurin in AML is 50 mg twice daily with food on days 8 to 21 of each cycle of induction and consolidation chemotherapy followed by 50 mg with food as a single agent for up to 12 months. The objective of this study was to evaluate the utilization of midostaurin in AML in the real-world setting in the first year after its US approval and marketing.
Methods: Patients with at least 1 claim for midostaurin (index date) between April 1, 2017 and October 31, 2018 and who had at least 1 claim with a diagnosis of AML in the 6-month pre-index period were identified from Symphony Health's Integrated Dataverse (IDV), a large US claims database containing linked longitudinal prescription, medical, and hospital claims. The IDV contains claims for 280 million active unique patients representing over 73% of specialty prescriptions, 58% of medical claims, and 30% of hospital claims volume in the US. Patients included in the study cohort had a 6-month pre-index period with no claims with a diagnosis of AML, were at least 18 years old at initiation of midostaurin, and no evidence of participation in a clinical trial. Patient characteristics and treatment patterns were summarized using descriptive statistics. Median and 95% confidence interval (CI) for duration of midostaurin were calculated using Kaplan-Meier estimates.
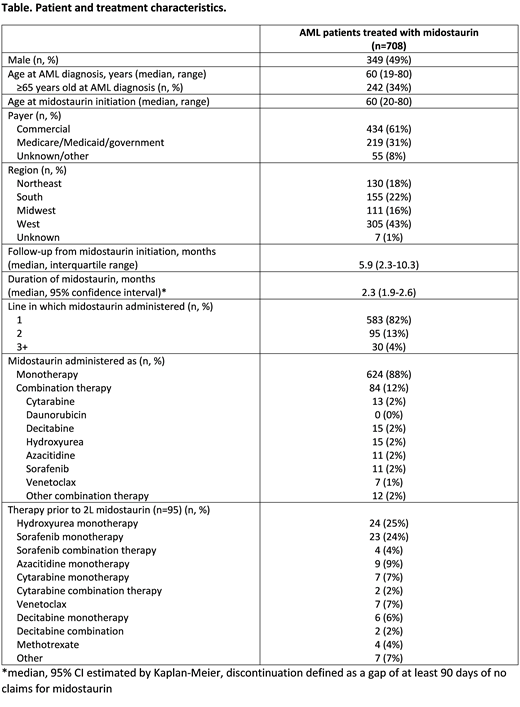
Results: There were 708 patients with AML treated with midostaurin who met all study selection criteria. Median age at midostaurin initiation was 60 years (range 20-80), and 49% of patients were male. The majority (61%) had commercial insurance, and patients resided in all 4 US regions (43% West, 22% South, 18% Northeast, 16% Midwest). Midostaurin was given as first line therapy in 583 (82%) patients, as second line in 95 (13%) patients, and as third line or later in 30 (4%) patients (see Table). Among the 95 patients who received midostaurin as second line therapy, hydroxyurea monotherapy (n=24, 25%) and sorafenib monotherapy (n=23, 24%) were most common first line therapies. With a median follow-up of 5.9 months from initiation of midostaurin, the median duration of midostaurin treatment was 2.3 months (95% CI 1.9-2.6). Over half (51%) had an average daily dose of 100mg on their first fill, 25% had 50mg, 9% had 67mg, 5% had 25mg, and 10% had other doses.
Conclusions: In this retrospective claims-based study, there is evidence of early uptake of midostaurin use in AML. However, majority of patients receive midostaurin as a single agent contrary to the approved schedule of initiating midostaurin concurrently with IC and with consolidation cytarabine. The median duration of midostaurin treatment was short at 2.3 months given that the recommendation is to continue it for 12 months after IC. Almost half the patients are started at a dose that is lower than the recommended dose, which may impact efficacy. Potential limitations of this dataset include a relatively short period of follow-up, retrospective design and low uptake post-marketing as some patients may have completed IC or missing data from midostaurin administered in the hospital. Barriers to the appropriate use of midostaurin in AML, if any, will need further exploration. Universal and timely testing for the FLT3 mutation is the essential first step to allow for including midostaurin as a part of IC for the appropriate utilization of this agent in FLT3 + AML.
Gajra:Cardinal Health: Employment. Klink:Cardinal Health: Employment. Kish:Cardinal Health: Employment. Chopra:Cardinal Health: Employment. Feinberg:Cardinal Health: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal