Background
The oral anti-apoptotic B-cell lymphoma 2 protein inhibitor venetoclax has shown strong activity in R/R AML in controlled clinical trials, and recently impressive results in treatment-naïve AML elderly patients with acute myeloid leukemia. However, limited data are available in the real-life setting.
Methods
This is a multi-center (n=4), retrospective study involving patients with treatment-naïve or Relapsed/Refractory (R/R) AML treated with Venetoclax in combination with HMAs. Data were collected after anonymous aggregation, in accordance with GCP and Helsinky declaration. Adverse events (AEs) were graded according CTCAE v4.03. Survival is estimated with Kaplan-Meyer method.
Results
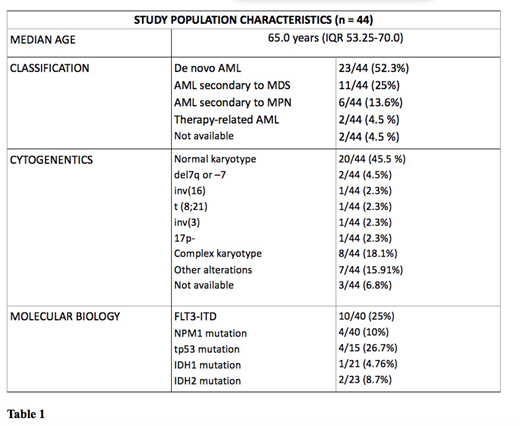
Forty-four patients have been prescribed Venetoclax from March 2018 to June 2019 and completed at least 1 course of venetoclax (range 1-8, median 2, IQR 2.0 - 4.0), being evaluable in this analysis. Patients's characteristics are summarized in Table 1. Five/44 (11.4%) patients had a low risk AML, 21/44 (47.7%) had an intermediate risk AML and 14/44 (31.8%) patients had a high risk AML, according to ELN 2017 risk stratification (4 patients had no available ELN risk at baseline). Six out of 44 (13.6%) patients received Venetoclax in combination with HMAs as first line of therapy, whereas 14/44 (31%) as first line rescue for resistant AML, 15/44 (34.1%) at first relapse, 9/44 (20.5%) for second or further R/R AML. Among R/R patients who received Venetoclax, 17/38 (44.7%) and 21/38 (55.2 %) had received chemotherapy or HMAs as induction therapy, respectively. Overall, Venetoclax was combined with azacitidine in 19/44 patients (43.2%), with decitabine in 19/44 patients (43.2%), with Low-dose of Cytarabine in 5/44 (11.4%), and was performed in monotherapy in 1/44 (2.3%) patient. Three out of 44 patients (6.8%) received a maximum dosage of 100 mg daily, 2/44 (4.5%) received 200 mg, 37/44 (84.1%) received 400mg and 2/44 (4.5%) received 600 mg. Fifteen out of 44 (34.1%) patients reduced the dosage of venetoclax for concomitant Azole administration. The median follow-up is 75.5 (IQR 45.2 - 178.5) days for patients who received upfront venetoclax therapy, while 143 (IQR 49.2 - 235.7) days for R/R patients. In the first-line setting, no patients reduced venetoclax dosage for concomitant adverse events; two neutropenia grade IV and two thrombocytopenia grade III have been documented. In the R/R setting, 14/38 (36.6%) patients reduced venetoclax dosage for concomitant adverse events. Specifically, we reported 22 adverse events, of which 10 were grade III-IV (5 neutropenia grade IV, 2 pancytopenia grade IV, 1 neutropenia grade III and 2 febrile neutropenia grade III). The overall CR rate is 16.7 % in newly-onset AML patients and 28.9 % in R/R patients, respectively. Two out of 6 treatment-naive patients had an evaluable response at 2 months after the beginning of Venetoclax treatment, and 2/6 had an evaluable 4-months response: 1 stable disease (SD) and 1 disease progression (PD) at 2 months,1 SD e 1 complete remission (CR )at 4 months.
Thirty-one out of 38 R/R patients had an evaluable response at 2 months and 21/38 had an evaluable 4-month response: 10 CR, 1 complete response with incomplete hematologic recovery (CRi), 14 SD and 6 PD at 2 months; 6 CR, 10 SD and 3 PD at 4 months have been documented.
After a short follow-up period (75.5 days), no patients who received Venetoclax as upfront therapy underwent an allogeneic hematopoietic stem cell transplantation (HSCT). On the other hand, after a longer follow-up period (143 days), 5 out of 38 patients (13.2%) received a HSCT after Venetoclax therapy among R/R patients. Median Overall Survival was not reached in the newly-onset cohort. In R/R setting, median OS was 253 days (95% C.I. 157-349).
Interpretation
These data extend to the real-life setting some previous evidence obtained from trials. In particular, our data confirm that venetoclax plus HMAs or LDAC has an acceptable toxicity profile and is safe and manageable. However, especially in the R/R setting, hematological toxicity represents the most frequent adverse event, arising some concerns about the optimal drugs management. Although our data suggest a similar clinical activity of venetoclax combinations to that reported in clinical trials, further studies from the real-life setting are highly warranted to confirm venetoclax efficacy under normal clinical practice.
GG and JN equally contributed
CP and AC equally contributed
Boccadoro:Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Mundipharma: Research Funding; Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Cavo:celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Papayannidis:Shire: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Teva: Honoraria.
Venetoclax is not approved to treat Acute Myeloid Leukemia in Italy
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal