Introduction. Prophylactic replacement of factor VIII (FVIII) is the gold standard for the management of severe hemophilia, preventing bleeding and its complications. One of the most commonly used methods in developing countries is the Swedish regimen that consist in the administration of 25-40 IU/kg of FVIII three times per week. However, the actual required levels may vary considerably, these depend on the dose administered, the dosage interval and the pharmacokinetic profile of the patient. Pharmacokinetic calculations based on bayesian estimation are useful for designing an optimal dosage of the minimum required factor levels for each individual patient, nevertheless in our country it is not accessible to all patients mainly due to the cost of the multiple samples needed to obtain the pharmacokinetic profile.
This report describes our experience determining the pharmacokinetics of FVIII in a cohort of pediatric patients with hemophilia A in order to establish a personalized prophylaxis and to compare the infusion frequency and factor consumption with the dosage regimen previously mentioned.
Methods. Pediatric patients with severe and moderate hemophilia A were included, with one or more years in prophylaxis and monthly follow-up at the hemophilia clinic of our centre. Patients with inhibitors and/or documented renal and/or hepatic alterations were excluded. We administered 50 U/kg of FVIII to all patients and 4 blood samples were collected for the estimation of FVIII phamacokinetics (0, 4, 24 and 48 hours after the application). FVIII was measured using one-stage assay. The FVIII pharmacokinetics of each patient were obtained using the WAPPS-Hemo app.
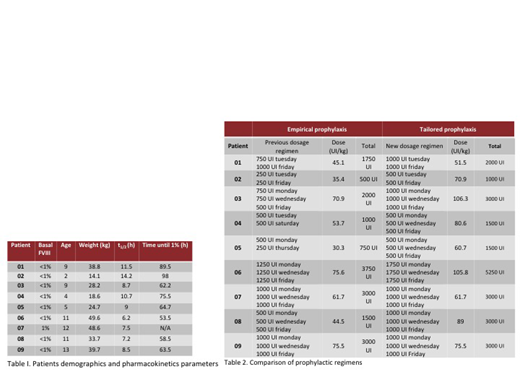
Results. The pharmacokinetics of FVIII were determined in 10 male patients (9 severe, 1 moderate), with a median age of 9 years (2-13) and a mean BMI of 18.6 (15.1-23.6). One patient was excluded for having an atypical t1/2 for FVIII, not within the population curve, in him the existence of an inhibitor was suspected and later corroborated. We observed an average estimated time of 70.6 hr (53.5-89.5) in which the FVIII concentration reached <1% and an average half-life of 9.3 hr (6.25-14.25). Using the clinical calculator of the application, a prophylactic regimen was obtained for each patient in order to maintain FVIII trough levels ≥1%. In 22.2% the factor dose was not modified and in the remaining 77.8% it was necessary to elevate the dose with an average increase of 23.2 IU / kg / week.
Conclusions. Tailored prophylaxis is easy to obtain with the currently available digital tools. In our study, unlike what is reported in previous similar studies, almost 80% of patients required an increase in the dose of factor according to their pharmacokinetics profile. We also found a clearance time lower than that reported in other populations groups maily based in adults cases, but very similiar to what was previously reported in a pediatric population from southern Mexico. Most of our patients had not reported clinically significant bleeding, but it is possible that they had had subclinical bleeding during the time they maintained a trough level of FVIII <1% with their previous prophylactic regimen. The impact of the implementation of prophylaxis by pharmacokinetics and its cost-effectiveness is currently being evaluated prospectively.
Disclosures No relevant conflicts of interest to declare.
Gomez-Almaguer:Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Teva: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal