Introduction: ADAMTS13 (A Disintegrin and Metalloprotease with ThromboSpondin type 1 repeats) activity remains a key tool in differential diagnosis of thrombotic microangiopathies (TMAs). However, ADAMTS13 testing is not readily available in many hospitals. Recently, PLASMIC and PLASIC scores have been developed to facilitate rapid recognition of TTP. We aimed to evaluate their usefulness compared to ADAMTS13 testing in a real-world cohort of TMA patients.
Methods: We enrolled consecutive patients with samples referred to our Center for ADAMTS13 measurement due to TMA over the last 2 years. Samples were collected either at first diagnosis or relapse before initiation of treatment. ADAMTS13 activity was measured with a commercially available and validated ELISA kit (Technozym, Diapharma). Clinical data were retrospectively collected from referring centers. Management was based on treating physicians' decisions.
TTP was defined as severe ADAMTS13 deficiency (activity≤10%); while secondary TMAs were diagnosed in patients with cancer, connective tissue disorders or hematopoietic cell transplantation recipients (transplant-associated TMA). Atypical hemolytic uremic syndrome (aHUS) remained a diagnosis of exclusion in patients with ADAMTS13>10%. PLASMIC was calculated based on seven variables: platelets, hemolysis, cancer, transplant, MCV, INR, creatinine; while MCV was not included in PLASIC, as previously described. ROC curve analysis was performed to determine the sensitivity and specificity of scores. Multivariate binary or logistic regression models were performed when appropriate.
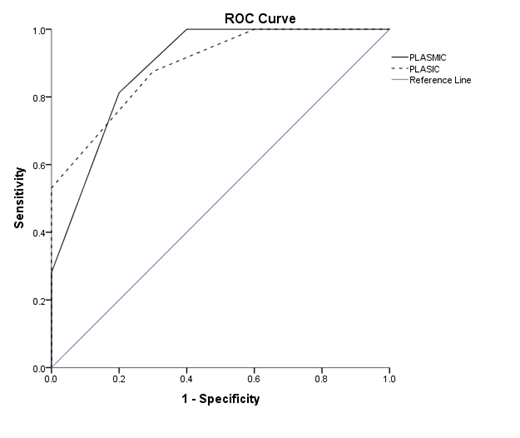
Results: We studied 50 TMA patients. Combined clinical and laboratory data conferred the following TMA classification: TTP in 36 patients (72%), transplant-associated TMA in 7 (14%), other secondary TMA in 5 and aHUS in 2. PLASMIC score was intermediate in 2 and high in another 4 patients without TTP. The PLASIC score was high in 5 patients without TTP, leading to less false positive results compared to PLASMIC (p<0.001). These patients suffered from secondary TMAs. In the ROC curve analysis, both PLASMIC and PLASIC significantly predicted TTP diagnosis (p<0.001 and area under the curve/AUC 0.891 and 0.892, Figure 1). In patients without secondary TMAs, PLASMIC and PLASIC had an excellent performance (p<0.001 and AUC 1).
Plasma exchange was commenced in the majority of patients (42/50, 84%). Among TTP patients, the majority (77%) received rituximab as salvage or prophylactic treatment. Rituximab administration was associated with platelet (p=0.003) and ADAMTS13 (p=0.015) levels at diagnosis. The complement inhibitor eculizumab was administered in 3 patients with TA-TMA, who achieved TMA resolution. With a median follow-up of 2.9 years (range 0.3-26.3), overall survival was significantly lower in patients with secondary TMAs (p<0.001). PLASMIC or PLASIC score were not associated with clinical outcomes in our cohort.
Conclusion: PLASMIC and PLASIC scores are excellent tools in TMA patients without secondary causes. While PLASMIC and PLASIC scores conferred similar outcomes, the PLASIC score requires six instead of seven variables, is classified as low/high omitting the intermediate category and leads to less false positive results. Further validation of the PLASIC score might confirm its clinical value. In addition, the role of ADAMTS13 levels in guiding rituximab administration needs to be further investigated. When an underlying etiology is detected, ADAMTS13 testing is necessary to exclude TTP and facilitate further therapeutic decisions.
Panayiotidis:Bayer: Other: Support of clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal