Introduction
Fostamatinib is a first in its class oral inhibitor of spleen tyrosine kinase (Syk) pathway for treatment of adults with immune thrombocytopenia (ITP) with insufficient response to previous treatment. Choice of treatment for relapsed ITP varies greatly according to clinician and patient preference. It is common for patients to switch between available thrombopoietin (TPO) agonists and now fostamatinib. Titration of these agents is often cumbersome and labor intensive; romiplostim in particular requires weekly visits with lab draws and consumes nurse, pharmacy and provider effort. Here we present real world experience with fostamatinib use in an academic medical center.
Methods.
We retrospectively identified four patients who were prescribed fostamatinib within the past nine months at Boston Medical Center Health System. All patients had a diagnosis of chronic ITP and were previously treated with corticosteroids, intravenous immunoglobulin (IVIG), and rituximab. With respect to previous TPO receptor use, two of four patients had received both eltrombopag and romiplostim. Patients were started on fostamatinib at a dose of 100 mg by mouth twice daily and titrated up to 150 mg by mouth twice daily if platelet counts were <50 10x9 /L after 4 weeks of therapy. All patients were seen by a pharmacist and provider for initiation of the drug and seen in clinic weekly for the first month on therapy to assess toxicity and efficacy. The duration of follow up for the patients is between 2 and 9 months on therapy.
Results.
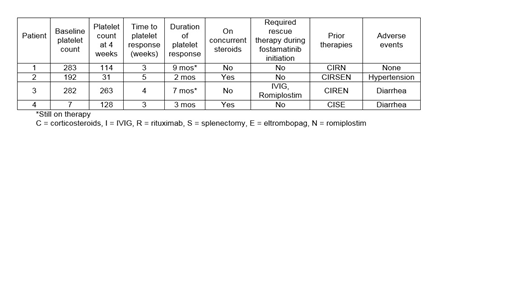
Three of four patients initiated on fostamatinib had a baseline platelet count 100K or greater and the fourth patient had ITP refractory to multiple lines of therapy with a baseline platelet count of 7K at therapy initiation. Three patients sought alternative therapy given side effects from TPO agonists or inconvenience of romiplostim. Three patients had platelets counts >50K at 4 weeks and one patient had a platelet count <30K. Recent platelet counts of the two patients that have been on therapy for 7 and 8 months were 123K and 108K, respectively. The third patient that responded initially discontinued therapy due to lack of stable response. Both of the patients with stable responses were well controlled on previous therapy with romiplostim. One patient required rescue therapy with IVIG and romiplostim due to petechiae 15 days after therapy initiation but continued on therapy thereafter. Two patients that discontinued therapy had platelet counts of 10K and 88K upon discontinuation due to inadequate response and toxicity, respectively. One patient was romiplostim naïve and the other patient had not received romiplostim for four years prior. Two patients developed diarrhea that was managed with loperamide and one led to treatment discontinuation (in combination with insufficient response). One patient experienced hypertension that was managed accordingly.
Conclusion.
The ideal place in therapy for fostamatinib in ITP therapy is not yet clear. However, the availability as an oral option for patients with refractory disease is appealing. One of our patients required rescue therapy which should be considered when transitioning patients to fostamatinib from TPO agonists. Additionally, side effect management with anti-hypertensives and anti-diarrheal agents may be required to continue therapy. Effectiveness of romiplostim may predict responsiveness to fostamatinib, although additional data are needed.
Shah:Rigel Pharmaceutical: Consultancy, Speakers Bureau. Sarosiek:Acrotech: Research Funding. Sloan:Merck: Other: endpoint review commitee; Abbvie: Other: Endpoint Review Committee; Stemline: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal