Background: Autologous T cells genetically modified with a lentiviral vector to express affinity-enhanced T cell receptors (TCR) or chimeric antigen receptors have shown great promise for the treatment of cancer. NY-ESO-1 is a cancer testis antigen with little normal tissue expression but with aberrant expression in MM, sarcomas, and melanomas. An HLA-A201 restricted TCR recognizing the NY-ESO-1/LAGE-1 157-165 epitope (SLLMWITQC) kills NY-ESO positive cell lines and has been used to treat 25 patients with MM after ASCT with expansion, persistence, antigen-directed functionality and long-term safety and antitumor activity (Nat Med 2015, Blood Adv 2019). We hypothesized removal of the genes encoding the endogenous TCR, TCRα (TRAC) and TCRβ (TRBC), would enhance NY-ESO TCR expression and reduce TCR mispairing and with removal of PD-1 (PDCD1) would enhance activity and persistence. We previously demonstrated CRISPR/Cas9 and TCRα, TCRβ and PDCD1 targeting gRNAs could be successfully introduced via electroporation in preclinical models to disrupt gene expression (Clin Cancer Res 2017). We therefore began a phase 1 pilot clinical trial for pts with advanced MM and sarcoma of NY-ESO-1 TCR-expressing T cells with CRISPR/Cas9 TCRα, TCRβ and PDCD1 edited genes to assess safety, feasibility and activity (NCT03399448).
Methods: Adults with HLA-A*0201 and expressing NY-ESO-1 and/or LAGE-1 antigen with advanced MM, synovial sarcoma, and myxoid/round cell liposarcoma (MRCL) with adequate performance and organ function and, for MM relapsed or refractory to at least 3 prior regimens and, for MRCL, proven metastatic disease or surgically inoperable local recurrence, were enrolled. Autologous T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC, TRBC and PDCD1 and subsequently transduced to express NY-ESO-1-specific TCR at the University of Pennsylvania. Frequency of NYCE T cells in final product was measured by flow cytometric dextramer analysis. Once cells were successfully manufactured and released, pts received fludarabine 30mg/m2 and cyclophosphamide 300mg/m2 daily on day -4,-3,-2. On Day 0 pts received a single infusion of thawed NYCE T cells as an out-patient. Pts were monitored closely for the first 28 days, monthly till 6 mo and then followed every 3 mo for adverse events, antitumor response and survival, NYCE T cell expansion, persistence, trafficking, phenotype and function, and immunogenicity. An assessment after accrual of the first 3 subjects in this ongoing trial was planned and is reported here.
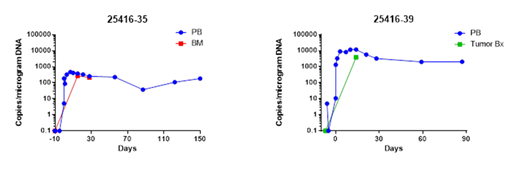
Results: 3 pts, 2 with MM and 1 with MRCL, have received NYCE T cells. Pt 1 is a 67 y/o F with IgG kappa MM with lytic bone lesions, and a +17q after 8 lines of therapy including 3 ASCTs, lenalidomide, pomalidomide, bortezomib, carfilzomib, daratumumab, and panobinostat. Pt 2 is a 65 y/o M with a recurrent MRCL manifested by abdominal and pelvic involvement after neo-adjuvant doxorubicin, multiple resections and radiation treatments with progression at time of enrollment. Pt 3 is a 62 y/o F with kappa light chain MM with lytic bone lesions and plasmacytomas and a +1q after 7 lines of therapy including lenalidomide, pomalidomide, bortezomib, carfilzomib, daratumumab, 2 ASCTs and an immunoconjugate . Manufacturing for these pts resulted in satisfactory products with 89.4 to 96% viability, transduction efficiency by qPCR of 0.04 to 0.2 copies/cell , residual Cas9 concentration 0 to 0.37 ng/ml, dextramer 0.4 to 1.8% NY-ESO-1 expression. TRAC, TRBC, and PDCD1 disruption efficiency was 44.3 to 49.4, 3.61 to 15.7 and 15.6 to 20.2% respectively. Pts tolerated treatment well without neurotoxicity or CRS. By day +60 pt 1 progressed by IMWG. Pt 2 received 1 U PRBC. By day +90 he remained with stable disease by serial CT scans. Pt 3 is too early to evaluate. Serial qPCR for copies of lentiviral transcripts in peripheral blood and tumor biopsies for pts 1+2 showed in vivo expansion, stable persistence and tumor targeting (Figure).
Conclusion: Early results of a phase 1 trial of NYCE T cells infused in 3 pts with advanced MM and MRCL show safety and feasibility and viable, expanding, and persisting CRISPR/Cas9 gene edited T cells that trafficked to tumor. The persistence of the NYCE T cells suggests that immunogenicity from multiplexed gene-editing using Cas9 is minimal under these conditions. Further characterization of phenotype and function of these cells and clinical outcomes will be presented.
Stadtmauer:Celgene: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Research Funding; Tmunity: Research Funding; Abbvie: Research Funding. Cohen:Poseida Therapeutics, Inc.: Research Funding. Lacey:Novartis: Patents & Royalties: Patents related to CAR T cell biomarkers; Tmunity: Research Funding; Novartis: Research Funding. Melenhorst:Incyte: Research Funding; Novartis: Research Funding, Speakers Bureau; Parker Institute for Cancer Immunotherapy: Research Funding; Genentech: Speakers Bureau; Stand Up to Cancer: Research Funding; IASO Biotherapeutics, Co: Consultancy; Simcere of America, Inc: Consultancy; Shanghai Unicar Therapy, Co: Consultancy; Colorado Clinical and Translational Sciences Institute: Membership on an entity's Board of Directors or advisory committees; National Institutes of Health: Research Funding. Fraietta:Tmunity: Research Funding; Cabaletta: Research Funding; LEK Consulting: Consultancy. Mangan:amgen: Speakers Bureau; takeda: Speakers Bureau; celgene: Speakers Bureau; janssen: Speakers Bureau. Lancaster:novartis: Research Funding. Suhoski:novartis: Research Funding. Fesnak:Novartis: Research Funding. Young:novartis: Research Funding. Chew:tmunity: Other: Scientific Founder, Research Funding; novartis: Research Funding. Zhao:Tmunity: Membership on an entity's Board of Directors or advisory committees, Research Funding; novartis: Research Funding. Hwang:Novartis: Research Funding; Tmunity: Research Funding. Hexner:novartis: Research Funding. June:Novartis: Research Funding; Tmunity: Other: scientific founder, for which he has founders stock but no income, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal