Background
Total pancreatectomy with islet autotransplantation (TPIAT) is a surgical treatment for chronic recurrent pancreatitis that involves splenectomy, pancreatectomy and creation of a Roux-en-Y, followed by re-injection of the pancreatic islets into the portal vein. Postoperatively, patients develop a sustained and extreme thrombocytosis (ExT) with platelets ≥1000 K/μL, a response more exaggerated than the typical post-splenectomy course (Gurria JP et al, Pancreas 2019). Empiric aspirin (antiplatelet effect) and hydroxyurea (cytoreduction) are initiated postoperatively per a standard protocol. The pharmacokinetic (PK) and pharmacodynamic (PD) profiles of hydroxyurea have not been studied in children other than young children with sickle cell disease; neither dose nor dose interval have been evaluated in TPIAT patients. Recently a population PK model was developed to support individualized hydroxyurea dosing in patients with sickle cell anemia (SCA; Dong M et al, Br J Clin Pharmacol 2016). In a prospective evaluation as part of the Therapeutic Response Evaluation and Adherence Trial (TREAT, McGann PT et al, Am J Hematol 2019), PK-guided individualized dosing resulted in better clinical and laboratory benefits than with conventional weight-based dosing. This study aimed to determine if a hydroxyurea PK model could be developed for non-SCA children dosed postoperatively for control of TPIAT-associated ExT, and if so, to compare it to previously published models for SCA.
Methods
A prospective single-site pilot study was performed in patients ages 0-21 years who underwent TPIAT between April 2018 and June 2019. Whole blood was collected via finger stick or venipuncture at 3 time points (20 minutes, 1 hour, 4 hours) after the initial hydroxyurea dose (15-20 mg/kg), given between postoperative day 5-7 (PK1). Plasma hydroxyurea was quantified by high performance liquid chromatography on 150-200 μL plasma. Testing was repeated once 2-6 months postoperatively (PK2) to determine whether PK profiles changed over time in relation to surgery. PK analyses and estimation of the area under the concentration-time curve (AUC) as a measure of exposure were performed using MW/Pharm (Mediware, Prague, Czech Republic). PK data from HUSTLE (NCT00305175) were used for comparison to SCA patients.
Results
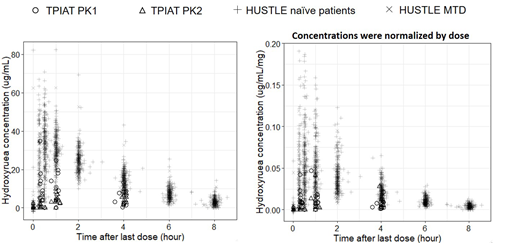
Of 19 enrolled subjects, 15 had evaluable results: 7 had both PK collections while 8 had a single collection (5 only had PK1, 3 only had PK2). All 5 patients with only PK1 measurements had discontinued hydroxyurea by the time PK2 samples would have been collected, whereas those with only PK2 all had unsuccessful PK1 collection attempts. Mean age was 13.5 years [standard deviation (SD) 5] with mean weight-based dose 14 mg/kg (SD 3.4). Serum creatinine was normal for age in all patients. Hydroxyurea suspension was delivered via jejunal tube at PK1 while oral tablets were used at PK2 per surgical protocol. The AUC was 60.1 h*μg/ml at PK1 and 68.6 h*μg/ml at PK2. When compared to HUSTLE cohort (Figure 1), TPIAT subjects overall had lower drug exposure (AUC), worse for PK1 than PK2, especially when hydroxyurea concentration was normalized to dose. TPIAT patients also appear to have had slower absorption, evidenced by still-rising hydroxyurea concentrations after 1 hour, compared to 20-30 minute peak concentrations in HUSTLE.
Conclusions
TPIAT subjects treated empirically with hydroxyurea to modulate ExT have decreased absorption and overall lower concentrations and drug exposure relative to our SCA population. Lower bioavailability and/or altered absorption may in part be caused by differences in formulation (suspension versus tablet) and route of administration (oral versus jejunal) along with postoperative anatomic changes not present in the SCA cohort, since Roux-en-Y may alter drug absorption and metabolism (Rogers CC et al, Clin Transpl2008). It is unknown whether higher enteral hydroxyurea doses would increase exposure and effect. Additional evaluation of the absorption characteristics and PK/PD of hydroxyurea in TPIAT patients, as well as efficacy, are urgently needed.
Ware:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Nova Laboratories: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: DSMB; Bristol Myers Squibb: Other: Research Drug Donation; Addmedica: Other: Research Drug Donation.
Hydroxyurea to reduce extreme thrombocytosis after splenectomy/pancreatic islet cell transplant in children
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal