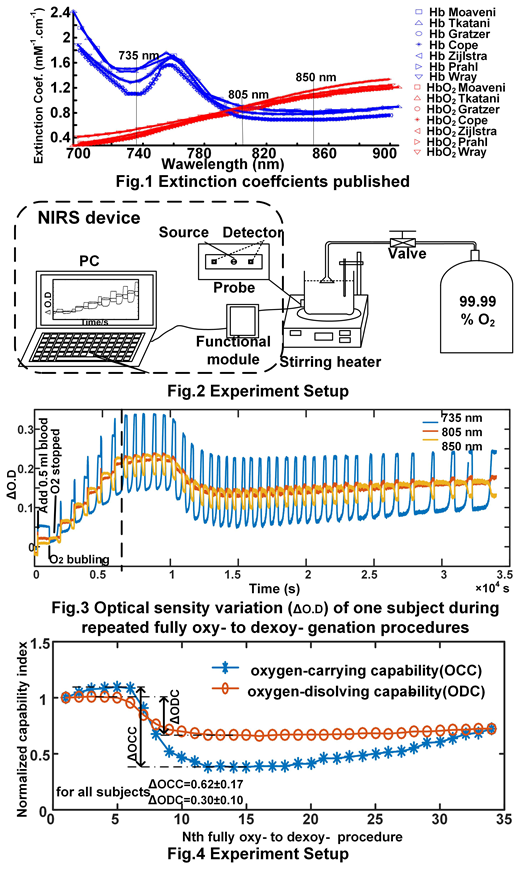
Oxygen dissolving and dissovling capabilities ( briefly, ODC and OCC) are very valuable to assess the functions in oxygen transport and metabolism of red cells, especially for research on, diagnosis, and evaluating therapeutic effects of sick cell disease, thalassemia, and even bone marroe failure. However, the approach to quantify ODC and OCC of red cells is sparse. Mostly, researchers and clicians used oxygen saturation by blood oxymeter to reflect OCC, not right though. ODC and OCC are more resonable to be assessed in extreme situations, e.g., fully deoxy- and oxy- genation. Near infrared spectroscopy (NIRS) [1] has been intensively developed for noninvasive meauring oxygenation changes these years, which utilized the oxy- and dexoy- hemoglobin senstive wavelengths to quantify concentration changes of Hb and HbO2 [2] ( fig.1 ). NIRS is of strong potential in noinavsive assessing blood oxygenation in deep muscle, breast, brain, and tumor [3]. Here we attempt to assess ODC and OCC of red cells to by use of NIRS with aid of a protocol with repeated fully oxy- to deoxy- genation process.
Blood samples were got from 8 healthy males and 5 healthy females ( 21.7±2.1 years old ) who were recruited from the university community by physical check-up. No subject had taken any drugs before 3 ml blood sampling. The experiment setup consisted of a tissue like liquid phantom, a NIRS probe [2], a function module and a computer, as shown in Fig.2. The phantom was prepared to simulate blood content and oxygen variation in living human tissue with similar optical property with human tissue, containg 450 ml PBS, 10 ml 10% intralipid solution serving as a scatterer, placed on the plate of a magnetic stirring device to control the solution at 37±1℃. Priorly, 5 g yeast, proved to be more than enough to dioxide the hemoglobin, was spread into the mixture solution. After 20 s of baseline measurement, 0.5 ml human blood was added into the solution to simulate blood content variation. Then, 99.99% oxygen gas, through a container-sized nozzle with dense holes transiting uniform high-pressure 25 MPa oxide, was lead into the solution to fully oxygenate the red cells. Then, the oxygen gas supply was stopped to allow oxygen to be consumed fully by the yeast. The same process, was repeated 6 times. Then, no blood added and repeat above cycle from 100% oxygenation to 100 deoxygenation 35 times. The data of 2 male and 1 female was exluded for solution spilling. The delta optical density ( ∆O.D ) of oxy- and deoxy- sensitive wavelengths were got by NIRS, as shown in Fig. 3 for example, Then we extract the ∆O.Ds at 100% oxygen carrying and 100% oxygen dissolving stages for the final no red cells added 35 process cycles.
Among all subjects, the ∆O.Ds curves respond to the repeated 35 cycles of fully oxy- to deoxy- genation at both oxy- and deoxy- sensitive nms formed a first stable then fast drop and then very slow recovery shape. The extracted normalized OCC curves formed higher in in fast drop phase than OCC extracted from above curves. For all subjects, ∆OCC in that phase ranged in 0.62 ± 0.17 while ∆ODC ranged in 0.30 ± 0.10. And there is a positive correlation between ∆OCC and ∆ODC with r = 0.821 and p<0.01.
Over all, by use of NIRS with aid of a protocol with enough times ( ≥35 ) repeated fully oxy- to deoxy- genation process, we achieved in assessing both capabilities of red cells in carring and dissolving oxygen ( OCC and ODC ). This novel approach is good to be used to study oxygen function of red cells quantitatively, as well as diagnozing and tracking therapeutic effects of blood diseases. The theory to get why the OCC dropt more than ODC in our protocal is worth of further study, which probabaly allow us to find a path to improve OCC for curing low OCC patients with blood deaseses, such as sick cell disease and thalassemia. And it is worth saying that this study paved a way for NIRS to assess OCC and ODC on patients in noninvase way in near future.
[1] F. F. Jöbsis, "Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters," Science, vol. 198, pp. 1264-1267, 1977
[2] Y. Zhao, L. Qiu, Y. Sun, C. Huang, T. Li, Optimal hemoglobin extinction coefficient dataset for near-infrared spectroscopy, Biomed. Opt. Express, vol.8, no.11, pp. 5151-5159, 2017
[3] T. Li, C. Xue, P. Wang, Y. Li, L. Wu, Photon penetration depth in human brain for light monitoring and treatment: A Realistic Monte Carlo Simulation Study, J. Innovative Opt. Health Sci., vol. 10, no. 6, pp. 1743002, 2017
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal