Objectives: Worsening of symptoms by oncology treatment can adversely affect QoL. The MD Anderson Symptom Inventory (MDASI) is a patient-reported outcome capturing the severity of symptoms and their interference with daily living (activity, working, relations with people, enjoyment of life, mood). The QoL impact of polatuzumab vedotin (pola) plus rituximab (R) was assessed by MDASI in patients (pts) with NHL.
Methods: We analyzed pts in the pola + R cohorts of the Phase I/II ROMULUS study (NCT01691898): 40 pts with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) treated with pola (2.4mg/kg) + R, 20 pts with R/R follicular lymphoma (FL) treated with pola (2.4mg/kg) + R, and 20 pts with R/R FL treated with pola (1.8mg/kg) + R. Pts were treated until progressive disease/unacceptable toxicity, up to 17 cycles. MDASI questionnaires were completed at baseline (BL) and Cycles 1-8. Pts rated the worst severity of their symptoms in the last 24 hours from 0 (not present) to 10 (worst imaginable). Interference with daily living was rated from 0 (did not interfere) to 10 (interfered completely). We classed symptom/interference severity as mild (<5), moderate (5-6) or severe (≥7). Total symptom score (SS; 13 core items), total symptom interference score (SIS; six items), individual symptom scores, and change in scores were reported as arithmetic mean (SD). With no literature consensus on clinically meaningful MDASI differences in lymphoma, we pre-specified a two-point difference as meaningful for this analysis.
Results: For DLBCL and FL (pooled doses) cohorts, questionnaire completion rates at BL were 50% (20/40) and 75% (30/40), respectively. Completion rates at Cycle 1-8 ranged from 47-74% (DLBCL) and 67-90% (FL); 10 DLBCL pts and 1 FL pt did not complete a questionnaire after baseline.For DLBCL and FL, symptoms were mild and interference was low at BL. For DLBCL, mean (SD) SS was 1.60 (1.49) and SIS was 2.90 (3.11). Most BL symptoms were rated 'mild' and interference with daily living was low; the most common severe symptoms were distress, constipation, and fatigue (each 20% of pts). The mean BL score for numbness/tingling was 1.25 (1.97; n=19 mild/n=1 severe). For FL (pooled doses), mean SS was 1.28 (1.21) and SIS was 1.95 (2.28) at BL. The most common moderate symptom was fatigue (23% of pts) and the most common severe interference was with work (17%). The mean BL score for numbness/tingling was 1.00 (1.93; n=28, mild/n=2, severe) in pts with FL.
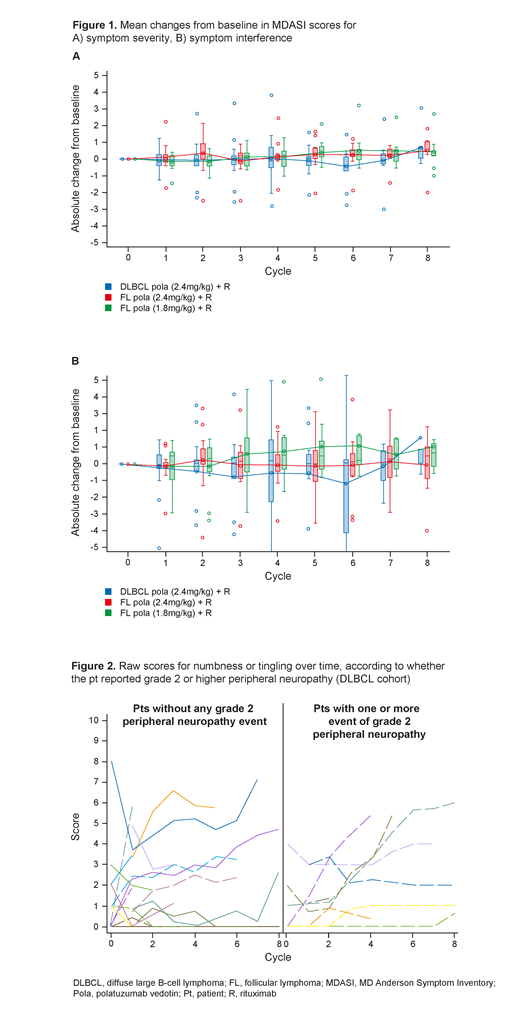
Fig 1 shows the SS and SIS change across cycles. For pts with DLBCL treated with pola (2.4mg/kg) + R, estimated mean changes from BL to Cycle (C) 6 and from BL to C8 were: -0.51 (1.26) and +0.67 (1.36), respectively, for total SS; and -1.21 (3.75) and +1.52 (3.35), respectively, for SIS. For pts with FL treated with pola (2.4mg/kg) + R, estimated mean changes from BL to C6 and BL to C8 were: +0.20 (0.75) and +0.47 (1.05) (total SS); and -0.12 (1.89) and -0.09 (1.83; SIS). For FL treated with pola (1.8mg/kg) + R, estimated mean changes from BL to C6 and C8 were: +0.48 (1.05) and +0.42 (1.02; SS); and +1.04 (2.07) and +0.98 (1.97; SIS). Similar results were obtained using a linear mixed effect model accounting for missing data.
For DLBCL, there was a slight increase in severity scores for numbness/tingling over time, with mean changes from BL of +0.99 (2.29) at C6 and +2.27 (2.39) from BL to C8. An increase from BL was observed for weakness in arms/legs (changes of +1.08 [2.08] from BL to C6 and +1.74 [2.87] from BL to C8). For FL (pooled), there was a slight increase from BL in numbness/tingling over time, with mean changes of +1.73 (1.92) at C6 and +2.55 (2.37) at C8. An increase was observed from BL in SS for weakness in arms/legs (+1.32 [1.92] from BL to C6 and +1.63 [2.72] from BL to C8, respectively). There was no clear association with numbness/tingling and peripheral neuropathy in DLBCL (Fig 2) or FL (not shown).
Conclusions: Symptom burden and interference with daily living remained relatively low for pts with R/R DLBCL or FL while on treatment with pola 1.8 or 2.4mg/kg + R, with few clinically meaningful changes in overall symptom and symptom interference scores. A slight increase was observed in scores for numbness and tingling, and weakness of arms/legs on treatment.
Thuresson:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Gentile:Genentech, Inc.: Employment. Ramies:Genentech, Inc.: Consultancy, Employment. Launonen:F. Hoffmann-La Roche Ltd: Employment. Morschhauser:BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria.
Polatuzumab vedotin (POLIVY, Genentech, Inc.) is a CD79b-directed antibody-drug conjugate. It was approved by the FDA in June 2019 in combination with bendamustine and rituximab for the treatment of adults with relapsed/refractory diffuse large B-cell lymphoma after at least two prior therapies.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal