Introduction: Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common types of cutaneous T-cell lymphoma (CTCL). Incidence rates for CTCL are higher in blacks than Caucasians (9.0 vs 6.4 cases per million persons). Retrospective studies suggest that black patients with CTCL present with younger age at diagnosis, lower male to female ratio, higher cutaneous disease burden, and inferior survival (Nath S, et al. Clin Lym Myel Leuk 2014). Prospective data on treatment outcomes in this patient subset are rare. Mogamulizumab, an anti-CCR4 monoclonal antibody, was recently FDA approved for relapsed/refractory (R/R) MF and SS with improved response rates and progression-free survival (PFS) vs comparator vorinostat. Here we report the clinical characteristics and outcomes of black patients treated in the MAVORIC trial with a specific focus on responses to mogamulizumab.
Methods: The MAVORIC trial was an international, open-label, randomized phase 3 trial comparing mogamulizumab vs vorinostat in R/R MF/SS. Complete study design and results were recently published (Kim YH, et al. Lancet Oncol 2018). Patients were randomized 1:1 and stratified by both clinical stage (IB-II vs III-IV) and disease type (MF vs SS). The primary endpoint was PFS, based on investigator assessment. Key secondary endpoints included overall response rate (ORR), duration of response, response by compartment (blood, skin, nodal, and visceral). We conducted a subset analysis on black vs non-black patients who were enrolled in the intention-to-treat population (ITT).
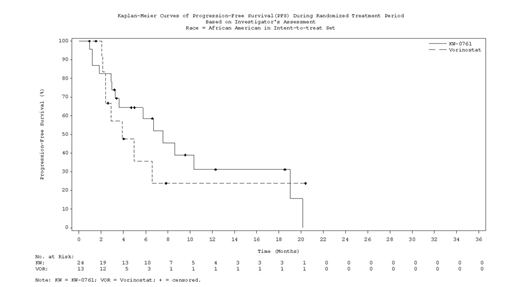
Results: 372 patients were enrolled of which 37 (10%) self-identified as black. Compared to non-black patients (N=335), the median age was younger (53 vs 66 years). Twenty-one (56.8%) were male, similar to 58.2% in non-black patients. Skin stage distribution in the black patients was: T1, N=0; T2, N=18 (48.6%); T3, N=4 (10.8%); and T4, N=15 (40.5%). Twenty-seven (73%) had blood involvement and 32 (86.5%) had lymphadenopathy. A greater fraction of the randomized black patients had MF compared to non-black (73% vs 52.8%); the opposite was true for SS (27% vs 47.2%). More black patients were randomized to mogamulizumab (N=24) than vorinostat (N=13). Median exposure to mogamulizumab was 170 days for all populations analyzed (black, non-black, and ITT). Instead median exposure to vorinostat was longer in black vs non-black patients (105 vs 83 days), and in blacks vs ITT (105 vs 84 days). Nearly two thirds (61.5%) of black patients randomized to vorinostat crossed over to mogamulizumab, slightly less than in the ITT population (73%). Median follow-up for black patients and for the entire MAVORIC cohort were 20.8 months and 17 months, respectively. In the ITT cohort, PFS was superior in patients randomized to mogamulizumab vs vorinostat (7.7 vs 3.1 months; HR 0.53; P<0.0001) (Kim YH, et al. Lancet Oncol 2018). In ad-hoc investigator assessed subgroup analyses, PFS also favored mogamulizumab over vorinostat in black patients [7.57 vs 3.9 months, respectively; HR 0.81; (95% CI 0.33-1.96)]. Additionally, by both investigator assessment and independent review, ORR to mogamulizumab was comparable to non-black patients. Similarly, mogamulizumab in black patients was superior to vorinostat in terms of responses by compartment with a confirmed response rate of 72.2% vs 11.1%, respectively in the blood, and a confirmed response rate of 41.7% vs 7.7%, respectively in the skin. Responses by compartment to mogamulizumab in non-black patients were similar (66% for blood and 42% for skin). There were fewer nodal responses in black patients (9.1%) compared with non-black patients (16.7%). When analyzed by disease subtype, higher ORR were seen in black patients randomized to mogamulizumab than to vorinostat for both MF (33.3% vs 11.1%, respectively) and SS (16.7% vs 0%, respectively). There were no differences in safety signals in black patients vs non-black. There was a slightly higher rate of infusion reactions 41.7% vs 31.9%. The rate of drug eruptions was similar, 25% vs 24.4%.
Conclusion: These results support mogamulizumab as an effective treatment for MF/SS in black patients with similar PFS and ORR, including by disease compartments (blood and skin) and responses by disease type (MF or SS), as non-black patients. Safety signals for black vs non-black patients were similar with mogamulizumab, including drug eruptions.
Porcu:Viracta: Honoraria, Other: Scientific Board, Research Funding; Kyowa: Honoraria, Other: Scientific Board, Research Funding; Innate Pharma: Honoraria, Other: Scientific Board, Research Funding; Daiichi: Research Funding; BeiGene: Other: Scientific Board, Research Funding; Incyte: Research Funding; Spectrum: Consultancy; ADCT: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal