Background: Rituximab increases the possibility of reactivation of hepatitis B virus (HBV) in patients with diffuse large B cell lymphoma (DLBCL), which is the most common non-Hodgkin lymphoma worldwide. Managing HBV during the treatment for DLBCL is therefore a particularly important issue in HBV endemic area, such as Taiwan. However, there is no population-based study to investigate whether HBV status and antiviral prophylaxis influence the survival in DLBCL patients.
Objective: To evaluate the impact of HBV status and antiviral prophylaxis on survival in DLBCL patients, we incorporated the data from the Taiwan Cancer Registry Database (TCRD), the National Health Insurance Research Database (NHIRD), and the National Death Registry in our study.
Methods:From TCRD, we identified 6304 patients with newly diagnosed DLBCL between 2011 and 2015. Patients were excluded if they were younger than 20 years (n=52), had unknown Ann Arbor stage (n=277), had other cancers before the diagnosis of DLBCL (n=336), or did not receive any treatment for DLBCL (n=514). We also excluded patients treated with regimens other than R-CHOP, R-CVP, CHOP, or CVP (n=711), with unknown HBV (n=492) or hepatitis C virus (HCV, n=152) status, or treated with antiviral therapies but not HBV or HCV carriers (n=65). There were 3702 DLBCL patients treated by R-CHOP, R-CVP, CHOP, or CVP for survival analysis, including 781 HBV carriers.
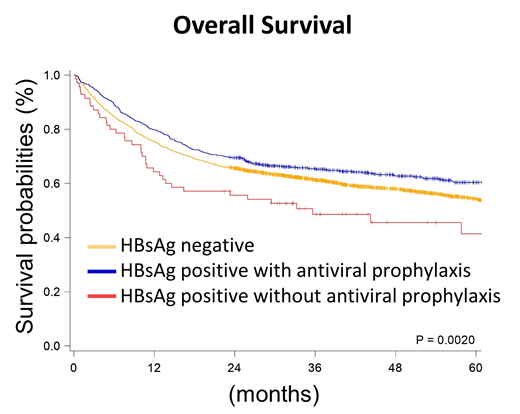
Results: According to the HBV status and the applications of antiviral prophylaxis, we stratified patients into three groups, HBsAg-negative patients (HBV-neg, n=2921), HBV carriers with antiviral prophylaxis (HBV+Tx, n=711), and HBV carriers without antiviral prophylaxis (HBV+No, n=70). The baseline characteristics were similar between the three groups, except that HBV+Tx patients were younger than others (median age: 63.7 years in HBV-neg patients, 57.0 years in HBV+Tx ones, and 62.9 years in HBV+No ones). HBV+Tx patients tended to receive R-CHOP, which was the standard frontline regimen for DLBCL patients (78.2% in HBV-neg patients, 87.5% in HBV+Tx ones, and 77.1% in HBV+No ones). The most frequent used antiviral agent was entecavir (91.0%). In survival analysis, the median overall survival (OS) of HBV-neg patients was 74.23 months, similar to that of HBV+Tx patients (median OS was not reached). However, the median OS of the HBV+No patients was only 35.61 months (P=0.0028 when compared with HBV+Tx patients), which indicated the antiviral prophylaxis improved OS in DLBCL patients with HBV infection. In the multivariate analysis of OS, we included other potential prognostic factors in DLBCL patients, such as gender difference, age, Carlson comorbidity index, Ann Arbor stage, type of frontline chemotherapies for DLBCL, radiotherapy in the frontline therapy, and the practice setting (medical centers or others). The status of HBV and antiviral prophylaxis was still an independent prognostic factor in the multivariate analysis of OS (hazard ratio and 95% confidence interval: 1 for HBV-neg, 1.01 (0.88-1.16) for HBV+Tx, and 1.70 (1.22-2.35) for HBV+No; P=0.0069).
Conclusion:From our population-based study, we illustrated the importance of antiviral prophylaxis in the DLBCL patients with HBV infections. HBV carriers would have similar survival to the HBV-negative patients if they received antiviral prophylaxis for HBV infections.
Tien:Daiichi Sankyo: Honoraria; Celgene: Honoraria; Pfizer: Honoraria; Abbvie: Honoraria; Johnson &Johnson: Honoraria; Novartis: Honoraria; Roche: Research Funding; Alexion: Honoraria; Celgene: Research Funding; Roche: Honoraria; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal