Introduction: For patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant-eligible, VTd with autologous stem-cell transplantation (ASCT) is a standard of care. In the PETHEMA/GEM study, pts in the VTd group received pre-ASCT induction therapy (six 28-day cycles) of bortezomib, thalidomide (ramp up to 200 mg daily, per label), and dexamethasone, followed by post-ASCT maintenance (up to 3 years) of interferon alfa-2b, thalidomide (100 mg daily), or thalidomide (100 mg daily) with bortezomib (1 cycle every 3 months; Rosiñol L, et al. Blood. 2012;120[8]:1589-96). Clinical practice has evolved and a modified thalidomide daily dose of 100 mg instead of 200 mg is now the standard of care (Mohty M, et al., presented at EBMT 2019, abstract OS12-3) and is reflected in treatment guidelines. In the phase 3 CASSIOPEIA study (part 1), pts received pre-ASCT induction (four 28-day cycles) and post-ASCT consolidation therapy (two 28-day cycles) of VTd (thalidomide 100 mg daily; VTd-mod) without or with daratumumab (D-VTd). In CASSIOPEIA part 2 (ongoing), pts with a partial response or better were re-randomized to maintenance therapy of daratumumab or observation (up to 2 years; Moreau P, et al. Lancet. 2019;394[10192]:29-38). Here, we used PSM to estimate the effect of treatments by controlling for differences in baseline covariates. We evaluated efficacy and safety in treatment groups from CASSIOPEIA versus the VTd group from PETHEMA/GEM (VTd-label).
Methods: Patient-level data for VTd-label were from PETHEMA/GEM, and VTd-mod and D-VTd were from CASSIOPEIA. Propensity scores were estimated using logistic regression. Pre- and post-match balance between groups was assessed using standardized differences for included covariates. The near neighbor matching procedure was used for matching. Covariates for matching were age, sex, ECOG performance status, myeloma type, International Staging System (ISS) disease stage, creatinine clearance, hemoglobin level, and platelet count. Outcomes observed in the matched sample were compared directly using a suitable measure of treatment effect for different endpoints. Outcomes of interest were overall survival (OS), progression-free survival (PFS), overall response rate (ORR) post-induction and post-ASCT, and rate of grade 3/4 peripheral neuropathy.
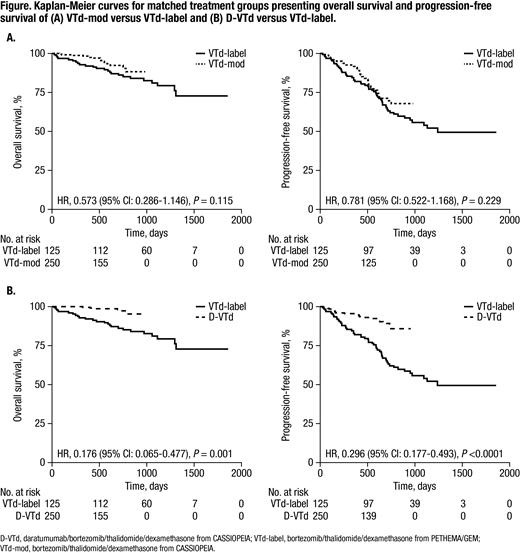
Results: Pts received VTd-mod (n=542), D-VTd (n=543) and VTd-label (n=130). After matching, 250 pts each for VTd-mod and D-VTd and 125 pts for VTd-label were included in the analyses. Baseline characteristics were similar across groups after matching. For OS and PFS, the comparison on matched samples demonstrated that VTd-mod was non-inferior to VTd-label (OS hazard ratio [HR] 0.573 [95% CI: 0.286-1.146], P=0.115; PFS HR 0.781 [95% CI: 0.522-1.168], P=0.229), and D-VTd was significantly better than VTd-label (OS HR 0.176 [95% CI: 0.065-0.477], P=0.001; PFS HR 0.296 [95% CI: 0.177-0.493], P<0.0001; Figure). Post-induction, the ORR for VTd-mod was non-inferior to VTd-label (odds ratio [OR] 1.314 [95% CI: 0.698-2.426], P=0.387). Post-transplant, VTd-mod demonstrated significantly better ORR versus VTd-label (OR 2.374 [95% CI: 1.316-4.292], P=0.004). D-VTd was significantly better than VTd-label for both post-induction and post-transplant ORRs (post-induction OR, 2.061 [95% CI: 1.050-4.036] P=0.034; post-transplant OR, 3.004 [95% CI: 1.626-5.624], P<0.0001). Compared with VTd-label, rates of grade 3/4 peripheral neuropathy were non-inferior for both VTd-mod (rate difference, 1.2 [95% CI: ⁻3.9-6.3], P=0.655) and D-VTd (rate difference, ⁻2.8 [95% CI: ⁻7.32-1.72]; P=0.186).
Conclusion: In this PSM analysis, non-inferiority of VTd-mod versus VTd-label was demonstrated for OS, PFS, post-induction ORR, and grade 3/4 peripheral neuropathy; VTd-mod was significantly better for post-transplant ORR. D-VTd was significantly better than VTd-label for OS, PFS, post-induction ORR, and post-transplant ORR, and was non-inferior to VTd-label for rates of grade 3/4 peripheral neuropathy. OS results for VTd-mod and D-VTd should be interpreted with the caveat that CASSIOPEIA OS data remain immature. These findings confirm those of CASSIOPEIA: D-VTd had superior efficacy versus VTd, both of which used a modified thalidomide dose. Potential imitations include unobserved confounding factors, and differences in study design (eg, response criteria and maintenance therapy).
Moreau:Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Hulin:celgene: Consultancy, Honoraria; Janssen, AbbVie, Celgene, Amgen: Honoraria. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hu:Ingress-health: Consultancy, Employment, Other: funding from Pfizer. Heeg:Ingress-Health: Employment. Hashim:Ingress-Health: Employment. de Boer:Janssen: Employment, Equity Ownership. Vanquickelberghe:Janssen: Employment, Equity Ownership. Kampfenkel:Janssen: Employment, Equity Ownership. Lam:Janssen: Employment, Equity Ownership. Cote:Janssen: Employment, Equity Ownership. Sonneveld:SkylineDx: Research Funding; Takeda: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Honoraria; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal