Background: After the introduction of reduced intensity conditioning (RIC) regimens, older patients became eligible for allogeneic hematopoietic stem cell transplantation (HSCT) with a parallel increase in the use of older sibling donors.
Clonal hematopoiesis of indeterminate potential (CHIP) is associated with older age and an increased risk of myeloid malignancies and cardiovascular complications. The impact of using older donors with CHIP on transplant outcomes have not been well-established.
In this study, we investigated the association between donor-CHIP and transplant outcomes in acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) patients who underwent HSCT from older donors (age >= 55).
Methods: Of 421 AML/MDS patients who underwent their first allogeneic HSCT from an older donor aged ≥55 from 2000- 2018, DNA samples were available from 363 healthy older donors at the time of stem cell donation. We performed targeted deep sequencing of 300 genes on donor blood samples and identified CHIP using modified Mutect and Pindel algorithms. We excluded variants with allele frequency < 2%.
After describing the distribution of CHIP among healthy older donors, we investigated the impact of donor CHIP on HSCT outcomes in a more homogenous population. We restricted clinical outcome analyses to 302 patients who underwent first allogeneic HSCT using a matched related donor (MRD), with peripheral blood stem cells (PBSCs) as the hematopoietic stem cell source. In this cohort, 90% of patients received tacrolimus/methotrexate for graft versus host disease (GvHD) prophylaxis.
Results: Among 363 donors, 71 (20%) were found to have CHIP. The most frequently mutated genes as CHIP were DNMT3A (32/71, 45%), TET2 (18/71, 25%), PPM1D (8/71, 11%), and SXL1 (7/71, 10%). Of 71 CHIP donors, 63 (89%) had single mutation, while 8 (11%) had two mutations.
There were no significant difference in recipients' disease status or transplant characteristics by donor CHIP status (Table 1). The presence of CHIP increased with donor age; the prevalence was 15% in donors aged 55-59, 16% aged 60-64, 32% aged 65-69 and 27% aged ≥70.
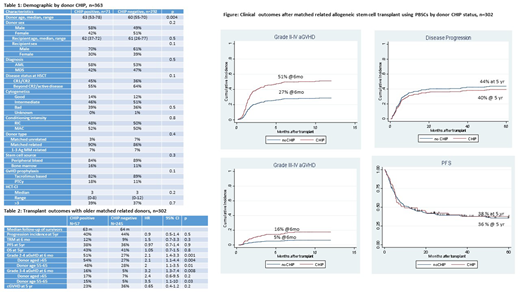
Transplant outcomes including engraftment rate, time to neutrophil and platelet recovery, progression incidence, progression-free survival (PFS) and overall survival (OS) were comparable by donor CHIP status (Figure) in the 302 patients with older MRDs and PBSCs as the stem cell source. Of note, disease and transplant characteristics were similar for comparison groups.
Cumulative incidence (CI) of grade 2-4 and 3-4 acute GvHD (aGvHD) at 6 months after transplant was higher with donor CHIP (HR=2.1, 95%CI=1.4-3.3, p<0.001 and HR=2.9, 95%CI=1.3-6.3, p=0.009) To further investigate the impact of donor CHIP on aGvHD, we investigated the interaction of donor CHIP with older donor age. Within healthy donors aged ≥65, CHIP increased the risk of grade 2-4 and 3-4 aGvHD (HR=2, 95%CI=1.1-3.5, p=0.01 and HR=3.5, 95%CI-1.1-10, p=0.03). The impact was similar in donors aged <65 with increased grade 2-4 and 3-4 aGvHD by CHIP (HR=2.1, 95% CI=1.1-4.4, p=0.04 and HR=2.4, 95%CI=0.6-9.5, p=0.2). Despite the increased aGvHD, we did not observe increased incidence of chronic GvHD by donor CHIP.
Conclusion: In this relatively homogenous population of AML/MDS patients who were transplanted after RIC with PBSCs from older matched related donors and who received tacrolimus-based GvHD prophylaxis, donor CHIP was associated with significantly increased risk of grade 2-4 and 3-4 aGvHD. Engraftment, progression risk and overall survival were not affected. Further studies to investigate the molecular mechanism of increased aGvHD and therapeutic interventions to improve aGvHD in the context of donor CHIP are warranted.
Oran:AROG pharmaceuticals: Research Funding; Astex pharmaceuticals: Research Funding. Champlin:Sanofi-Genzyme: Research Funding; Actinium: Consultancy; Johnson and Johnson: Consultancy. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kantarjian:Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding; BMS: Research Funding; Jazz Pharma: Research Funding; Novartis: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Ariad: Research Funding; Daiichi-Sankyo: Research Funding; Astex: Research Funding; Cyclacel: Research Funding; Immunogen: Research Funding; Pfizer: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Popat:Jazz: Consultancy; Incyte: Research Funding; Bayer: Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal