Introduction: Clinical practice guidelines recommend prophylaxis with granulocyte colony-stimulating factor (G-CSF) for most patients receiving chemotherapy with an intermediate risk or high risk for febrile neutropenia (FN). In real-world settings, however, many of these patients do not receive G-CSF prophylaxis, while other patients receiving low-risk or unclassified chemotherapy regimens receive G-CSF prophylaxis. Evidence on the effectiveness of G-CSF prophylaxis among diverse patient populations based on clinically rich data sources is currently limited.
Methods: A retrospective cohort design and data from four US health systems-Henry Ford Health System, Kaiser Permanente Northwest, Reliant Medical Group, and Geisinger Health System-were employed. Data included patients' demographics and clinical profile, cancer, treatment, supportive care, and outcomes, and were compiled from electronic medical records systems, administrative data warehouses, medical charts, and cancer registries. The study population comprised all patients who received a course of myelosuppressive chemotherapy for breast cancer, colorectal cancer, lung cancer, or non-Hodgkin's lymphoma (NHL) from 2009-2017. For each patient, each cycle of chemotherapy during the course, and use of G-CSF primary/secondary prophylaxis and FN events in each cycle, were characterized and included as a separate observation in the analytic file; thus, each patient could contribute multiple chemotherapy cycles to the analysis. Odds ratios (OR) for FN in cycle 1 and all cycles, respectively, were estimated for patients who did versus did not receive G-CSF prophylaxis in the corresponding cycle, and odds ratios were adjusted for clustering (by patient and health system [i.e., across cycles]) as well as for differences in baseline characteristics between G-CSF prophylaxis groups using inverse probability of treatment weighting (IPTW).
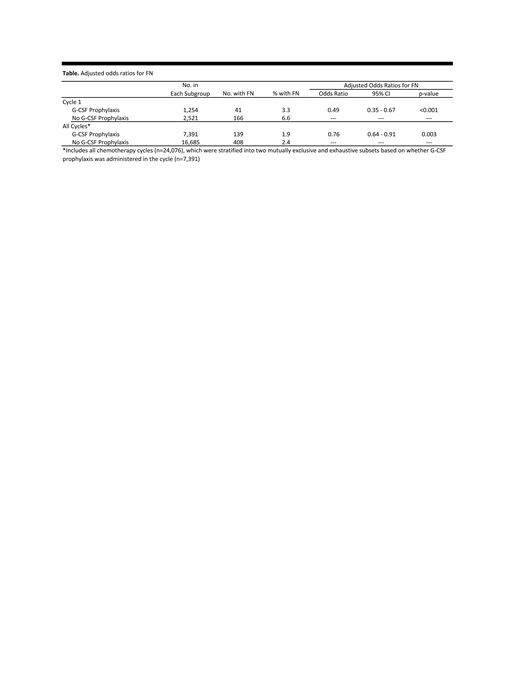
Results: The study population included 3,775 cancer chemotherapy patients who contributed 24,076 cycles of observation. The distribution of patients by cancer was: breast, 47%; lung, 26%; colorectal, 17%; and NHL, 10%; the distribution of patients by chemotherapy FN risk level was: high, 54%; intermediate, 12%; low, 15%; and unclassified, 19%. In cycle 1, 33% of patients received G-CSF prophylaxis (filgrastim, 53%; pegfilgrastim, 47%), and odds of FN in this subgroup were one-half those of patients who did not receive G-CSF prophylaxis (OR = 0.49 [0.35-0.67]) (Table). Across all cycles, 31% of patients received G-CSF prophylaxis (filgrastim, 55%; pegfilgrastim, 45%), and FN odds in these cycles were comparably reduced (OR = 0.76 [0.64-0.91]). Findings considering other model specifications, and across subgroups of the study population, were similar.
Conclusions: In this retrospective evaluation of patients receiving myelosuppressive chemotherapy for breast cancer, colorectal cancer, lung cancer, or NHL in US clinical practice, about one-third of patients received G-CSF prophylaxis in cycle 1 and subsequent cycles, and FN incidence was significantly lower among patients who did (versus did not) receive G-CSF prophylaxis. These findings highlight the effectiveness of G-CSF prophylaxis in reducing FN risk across cycles of chemotherapy among a diverse population of cancer patients receiving myelosuppressive regimens.
Lamerato:National Cancer Institute, Centers for Disease Control and Prevention, Amgen Inc., AstraZeneca, Evidera, eMAX Health: Research Funding. Lyman:G1 Therapeutics, Halozyme Therapeutics, Partners Healthcare, Hexal, Bristol-Myers Squibb, Helsinn Therapeutics, Amgen Inc., Pfizer, Agendia, Genomic Health, Inc.: Consultancy; Amgen Inc.: Other: Research support, Research Funding; Janssen Scientific Affairs, LLC: Research Funding; Generex Biotechnology: Membership on an entity's Board of Directors or advisory committees. Kaur:Amgen Inc., Evidera, eMAX Health: Research Funding. Shah:Amgen Inc.: Employment, Equity Ownership. Lawrence:Amgen Inc.: Employment, Equity Ownership. Silvia:Amgen Inc.: Research Funding. Hanau:Amgen Inc.: Research Funding. Weycker:Amgen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal