Introduction:
The gut microbiome is an important mediator of the host immune system, and studies in allogeneic transplantation demonstrate that loss of microbiome diversity is associated with inferior outcomes (Taur et al, Blood 2014). Loss of gut microbial diversity following autologous stem cell transplantation (ASCT) in multiple myeloma (MM) patients has been associated with reductions in progression-free survival but lacks clear validation from independent transplant centers, and the factors driving these changes are poorly understood (Khan et al, Blood 2018). Evidence from non-oncologic studies suggest that dietary factors and antibiotic exposure may play a key role in understanding the gut microbiota changes observed in the peri-transplant setting (Falony et. al., Science, 2016).
Methods
We performed a prospective study of changes to the gut microbiome following ASCT in 30 patients with MM using melphalan 200mg/m2 conditioning. Stool samples were collected at 4 timepoints: pre-transplant, post-transplant at engraftment, day +100, and day +365. Samples were processed within 48 hours and stored at -80C until undergoing DNA extraction and 16S rRNA sequencing. Microbiota analysis was performed using qiime and R programming software for species classification (using a pre-trained Silva database) and diversity calculations. Dietary analysis was performed prior to ASCT and at day +100 using the online diet history questionnaire version 3.0 (DHQ-3), a NIH-validated, web-based dietary survey that calculates daily nutrient consumption based on intake. We present dietary and demographic results of the entire cohort prior to transplant and microbiota changes in the pre-transplant and engraftment samples for the first 7 subjects.
Results
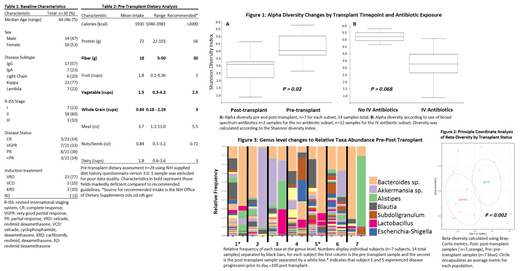
We enrolled 30 patients between 10/2018-6/2019. All patients received uniform infectious prophylaxis including levofloxacin from onset of neutropenia to engraftment or neutropenic fever, acyclovir, and fluconazole. Neutropenic fever was treated with combination vancomycin and cefepime until culture results could guide directed therapy or neutrophil engraftment. Demographics are displayed in table one and reflect a typical transplant-eligible myeloma cohort. DHQ-3 analysis (table 2) revealed deficiencies in fiber, vegetable, and whole grain intake below national guidelines, and female patients demonstrated significantly lower mean caloric intake compared to males (1679 vs 2015 calories/day, P= 0.047). Younger patients aged less than 60 demonstrated higher fiber intake compared to those aged 60 or older (19 vs 16 g/day P= 0.07) although this was not statistically significant. Microbiota changes revealed a significant loss of alpha diversity (P = 0.02) following transplant (figure 1), which was confirmed in a beta-diversity analysis demonstrating that pre- and post-transplant engraftment samples feature distinct microbiota communities (P = 0.002, figure 2). Diversity changes appeared to vary by exposure to antibiotics used for neutropenic fever, although this result was not statistically significant likely due to the limited sample analyzed at this time ( P = 0.068, figure 1). Two out of the 7 patients with available microbiota data demonstrated early disease progression before day 100, and analysis of the taxa changes at a genus level demonstrates a significant bloom of Bacteroides species at engraftment in these subjects (figure 3).
Conclusions
MM patients undergoing ASCT develop a significant loss of gut microbiome diversity following transplant, which confirms findings observed in other transplant centers. Further analysis of our cohort may determine if antibiotics significantly impact post-transplant diversity. To our knowledge, this is the first investigation to report pre-transplant dietary information in a microbiome study in ASCT, which may be important in understanding microbiome differences between patients and therapies and may guide future investigations. Prior to ASCT, most subjects were deficient in fiber intake, which may have important consequences to the gut microbiome as fiber is a key nutrient source for beneficial microbial species. Updated results from longitudinal study of our cohort will determine the potential impact of dietary intake on microbiome changes, and in turn, how these changes may affect clinical outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal