Introduction
Alemtuzumab is a monoclonal anti-CD52 antibody, a pan-lymphodepleting immunosuppressive agent in common use as part of conditioning for allogeneic stem cell transplantation (Allo-HSCT) in United Kingdom and many other centres across the globe, with benefits related to reduced graft versus Host disease (GVHD) and lower non-relapse mortality (NRM). However, evidence for effective dose schedule in Allo-HSCT remains debatable with some concerns related to delayed immune reconstitution and increased relapses with higher dosages; but increased risk of acute and chronic GVHD observed with lower doses. We present a large single-centre UK experience evaluating differential dosage effect of Alemtuzumab on HSCT outcomes.
Methods:
We retrospectively evaluated 330 patients undergoing Allo-HSCTs for myeloid malignancies (AML/MDS/MPNs) during a 10-year period (Jan 2010 to April 2019) at King's College Hospital, London. Two dosage schedules of Alemtuzumab based T-cell deplete conditioning regimen using 100mg (n-96) were compared to those receiving 60mg (n-234; <100mg dose) with respect to HSCT outcomes. Alemtuzumab based T deplete conditioning regimens included Fludarabine(Flu)-Busulphan(Bu) (2 doses as reduced intensity) while Flu-Bu4 or Bu4-Cyclophosphamide used as myeloablative protocol. Standard supportive care for GVHD prophylaxis (ciclosporin), viral/bacterial and anti-fungal prophylaxis was used. Close monitoring for infections, GVHD, chimerism (included fractionated lymphoid/myeloid) and disease assessments post HSCT were undertaken as per institutional policy. The data was compared and statistically analysed using Log-rank test for overall survival (OS), Cox regression for adjusted multivariate analysis and Gray method for cumulative incidences for NRM and relapses.
Results
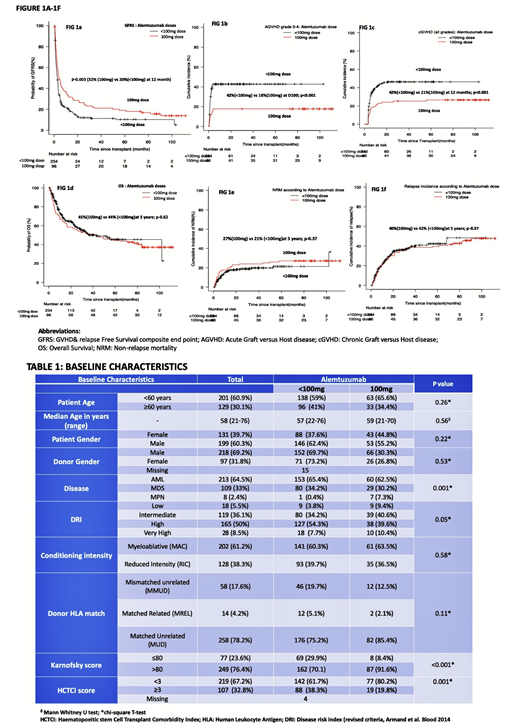
Baseline characteristics (Table-1) were similar between the 2 groups in terms of conditioning intensity, patient age, underlying disease (AML/MDS), disease risk and donor HLA matching. Median follow up of survivors was 34 months (range 01-112months) with significantly longer follow up available for 100mg dose group (median 90 months vs 28 months; p<0.001).
Higher Alemtuzumab dosage (100mg) was associated with a significant improvement in GVHD & Relapse free survival (GRFS)(32% vs 20% at 12 months;p-0.003; Fig 1a) and significantly lower incidence of both grade 3-4 acute (18% vs 42% at D100;p-<0.001; Fig 1b) and chronic GVHD (all grades)(21% vs 42% at 12 months; p<0.001; Fig 1c) compared to <100mg dose. No differences in OS (66% vs 71% at 12 months; p-0.62; Fig 1d), NRM (17% vs 16% at 12 months; p-0.37; Fig 1e) and relapse incidences (24% vs 24% at 12 months;p-0.71; Fig 1f) were observed with no impact on rates of CMV/EBV reactivation, disseminated Adenoviraemia or invasive fungal disease(IFD) between the 2 cohorts.
Multivariate adjusted cox analysis (MVA) confirmed older age>60 years, mismatched unrelated donor, absence of chronic GVHD, disease relapse, ITU admission event, CMV reactivation and absence of any EBV reactivation post HSCT as significant predictive factors for poor OS. This was not affected by different Alemtuzumab dosages, disease risk index or type of disease. However, GFRS was positively influenced by standard 100mg Alemtuzumab dose (HR 0.67; 95%CI: 0.51-0.86; p-0.005), no ITU admission event (HR-0.51; 95%CI:0.38-0.70; p<0.001) and EBV reactivation (HR-0.67; 95%CI:0.52-0.86; p-0.02) on multivariate analysis. NRM was worse for patients admitted in ITU, no known chronic GVHD, mismatched unrelated donor, presence of CMV and absence of EBV reactivation (p<0.001), but not affected by differential alemtuzumab dosages.
Conclusions
With improved supportive care, effective infection management and pre-emptive cellular therapy approaches available in current era, standard dosage (100mg) of alemtuzumab is safe and effective in both RIC and myeloablative allo-HSCTs for myeloid malignancies, with significantly lower GVHD related morbidity and overall better GFRS. Despite concerns of relapses and delayed immune reconstitution, this report on a homogenous cohort of allo-HSCTs in myeloid malignancies confirms the contrary with no impact on OS, NRM or relapses and no significant increase in opportunistic infections with 100mg dose.
Mufti:Celgene: Consultancy, Research Funding. De Lavallade:Bristol Myers Squibb: Research Funding; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal