Background:
Data suggests that the presence of measurable residual disease (MRD) at the time of transplant for AML portends a poor prognosis. The timing of MRD assessment and transplant relative to the amount of pre-transplant therapy, however, may affect this prognosis. To examine further the question of optimal timing of transplant in the setting of MRD, we reviewed outcomes in AML patients treated with traditional cytotoxic induction at our center who achieved CR1 and proceeded to transplant.
Methods:
We analyzed outcomes in patients undergoing first transplant for AML in CR1 between January 2014 and March 2018. CR was defined according to 2017 European Leukemia Network (ELN) guidelines. Non-core binding factor patients were included if they underwent initial therapy with 7+3 chemotherapy (+/- adjunctive inhibitor during induction or as post-transplant maintenance).
MRD testing modalities included cytogenetics/FISH (performed in 37 patients, positive in 10), flow cytometry performed by Hematologics Inc (performed in 49 patients, positive in 16), and/or mutation specific droplet digital PCR (ddPCR) performed at our center (performed in 33 patients, positive in 16). For three patients, other molecular methods demonstrated MRD.
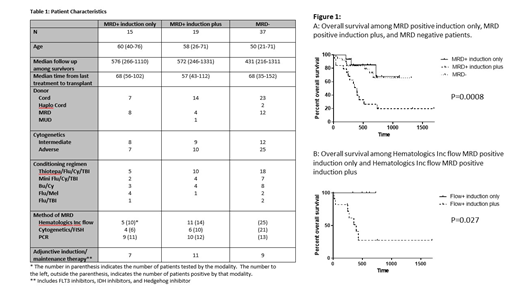
We compared outcomes in three groups: patients who underwent transplant in CR1 with positive MRD after induction only (MRD positive induction only) (n=15), patients who underwent transplant in CR1 with positive MRD after induction and additional therapy (MRD positive induction plus) (n=19), and patients undergoing transplant in CR1 with no MRD (MRD negative) (n=37). Patient details are summarized in Table 1.
Results:
CI of relapse was higher among MRD positive induction plus patients than MRD positive induction only patients (p=0.042, HR 0.301 (0.094-0.96)) and comparable among MRD positive induction only patients and MRD negative patients (p=0.987, HR 1.011 (0.29-3.58)). CI of transplant related mortality (TRM) was comparable between MRD positive induction plus patients and MRD positive induction only patients (p=0.165, HR 0.23 (95% CI 0.028-1.81)) and comparable among MRD positive induction only patients and MRD negative patients (p=0.871, HR 1.22 (95% CI 0.12-12.87)). Relapse free survival (RFS) and overall survival (OS) were comparable between MRD positive induction only patients and MRD negative patients and significantly better than for MRD positive induction plus patients (RFS (p<0.0001) and OS (p=0.0008)). (Figure 1) On multivariate analysis including MRD positive induction only, MRD positive induction plus, MRD negative, patient age, Sorror comorbidity index, donor source, conditioning regimen, use of adjunctive therapy, and ELN AML risk status, MRD positive induction plus was associated with a significantly higher risk of relapse than MRD positive induction only (p=0.014, HR 0.231 (95% CI 0.072-0.742)). No other factors were statistically significant.
Because multiple strategies were used to assess for MRD, we compared MRD positive induction only to MRD positive induction plus patients using flow cytometry performed by Hematologics Inc as the only MRD assessment technique. CI of relapse trended toward lower in the MRD positive induction only group (p=0.10, HR 0.22, (95% CI 0.036-1.34). OS and RFS were significantly improved in the MRD positive induction only patients (p=0.027 and p=0.026 respectively).
Conclusions:
For patients achieving CR following induction and moving directly to transplant in spite of MRD positivity, outcomes were comparable to patients going to transplant in an MRD negative state and were significantly improved compared to outcomes of patients in an MRD positive state who received additional therapy following induction.
Our series is small, and multiple MRD monitoring strategies were used. However, given the paucity of data on this specific question, uncertainty about whether MRD will clear with additional cytotoxic therapy following induction, and the poor prognosis of patients with persistent MRD in the induction plus group, we consider transplant following induction reasonable in this population regardless of MRD status. Larger series are necessary to more definitively answer this question.
Loken:Hematologics, Inc: Employment, Equity Ownership. Pollyea:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Diachii Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal