Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for pediatric patients with hematologic malignancies. Historically, matched sibling donors (MSD) have been the preferred donor source given ease of availability and lower rates of graft-versus-host-disease (GVHD). However, only 30% of patients have a MSD and relapse rates are high after MSD HSCT, raising the question of best donor choice. As conditioning regimens evolve, GVHD management improves and supportive care advances, it is important to evaluate the role of donor source on short and long-term clinical outcomes to inform donor selection. We performed a single-center retrospective analysis comparing post-HSCT outcomes in a cohort of pediatric patients undergoing MSD, matched unrelated donor (MUD), and umbilical cord blood (CB) transplants from 2006 to 2018.
Methods: A retrospective analysis was performed on an IRB-approved protocol through Fred Hutchinson Cancer Research Center. 232 patients were included who received MSD (n=56), MUD (n=89) or CB (n=87) transplants. Of note, 24 CB patients received expanded CB cells in addition to unmanipulated unit(s). GVHD prophylaxis in all patients consisted of a calcineurin inhibitor and MMF or methotrexate. The vast majority received a high-intensity conditioning regimen (86%, 96%, and 82% respectively for MSD, MUD and CB). Overall survival (OS) and disease-free survival (DFS) were evaluated using the Kaplan-Meier method. Probabilities of non-relapse mortality (NRM), relapse, and acute GVHD were evaluated using cumulative incidence (CI) estimates with appropriate competing risks. The Cox regression model was used for adjusted analysis for age, year of transplant, sex, CMV status, MRD status, disease risk, and conditioning regimen.
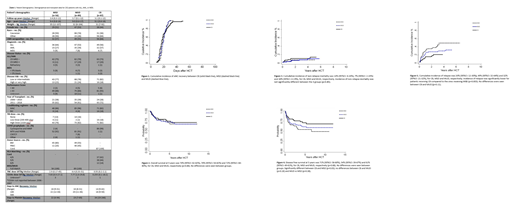
Results: Patient/treatment/donor demographics are shown in Table 1. Median follow-up was 2.6, 3.7 and 3.1 years for MSD, MUD and CB respectively. Patient diagnosis, disease risk, gender, age, and CMV serology were balanced between groups. CI of engraftment was similar as well, with only one graft failure in the MUD group (Fig 1). Median time to platelet recovery was significantly faster in MUD and MSD groups as compared to the CB group (p<0.0001; p<0.0001). There was no difference in unadjusted 5 year OS (Fig 2) and NRM (Fig 3) between groups. Five-year DFS was significantly higher in CB v. MSD (71% v. 54%, p=0.03) but not between CB v. MUD (71% v. 61%, p=0.18) or between MSD and MUD (p=0.38) (Fig 4). The CI of relapse at 5 years was 20% for CB patients, significantly lower than that of MSD recipients (49%, p=0.003) and not different from MUD (32%, p=0.11) (Fig 5). After adjusted analysis, the risk of DFS failures was higher among recipients of MSD than CB [HR: 1.88 (CI 95%: 1.01-3.47), p=0.04]. When CB versus MUD groups were compared, there was a trend of higher risk of DFS failures in the MUD group [HR: 1.28 (CI 95%: 0.96-1.66), p=0.09]. No difference was observed in risk of DFS failures between MSD versus MUD groups [HR:0.77 (CI 95%: 0.44-1.4), p=0.43]. Incidence of grade II-IV acute GVHD was 90% (95%CI: 82-95%), 75% (95%CI: 61-84%) and 88% (95%CI: 80-94%), for CB, MSD and MUD, respectively (p=0.25). Incidence of grade III-IV acute GVHD was 33% (95%CI: 23-43%) for CB, 9% (95%CI: 3-18%) for MSD, and 11% (95%CI: 5-18%) for MUD. Incidence of grade III-IV was significantly higher for the CB group compared to the other groups (p=0.001); however, nearly 60% of CB recipients with grade III-IV acute GVHD were diagnosed before engraftment had occurred and in retrospect met criteria for pre-engraftment syndrome. Among surviving patients, 23 CB recipients developed chronic GVHD (26% - 15 mild, 4 moderate, 4 severe) as compared to 14 MSD patients (25% - 13 mild, 1 moderate) and 40 MUD patients (45% - 27 mild, 11 moderate, 2 severe).
Conclusions: Our data demonstrate no difference in unadjusted OS between MSD, MUD and CB recipients. Importantly, despite this equivalence, 5-year DFS was significantly better in the CB v. MSD group, reflecting the lower relapse rate observed in CB patients and seen previously by us and others. CB continues to be viewed as an "alternative" donor for HSCT due to the low stem cell dose in a CB graft resulting in delayed neutrophil recovery, primary graft failure and increased NRM. However, this was not observed herein, supporting the use of CB for pediatric HSCT perhaps especially in patients at high risk of post-transplant relapse.
Milano:ExCellThera: Research Funding; Amgen: Research Funding. Delaney:Nohla Therapeutics: Employment, Equity Ownership; Biolife Solutions: Membership on an entity's Board of Directors or advisory committees. Bleakley:HighPass Biotherapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal