Introduction:
In addition to other disease-, patient- and procedure-related factors, recent studies revealed a strong impact of somatic mutations on the outcome after allogeneic transplantation (allo-SCT) in patients with myelodysplastic syndromes (MDS) and secondary acute myeloid leukemia (AML). The choice of pre-transplant strategy (upfront transplantation [Tx] vs. induction chemotherapy [CTX] vs. hypomethylating agent [HMA]) is a matter of debate among experts but may also influence post-transplant outcome. The issue whether there is an interplay between mutational profile and pre-transplant therapies and how this may modulate outcome after allo-SCT has not been investigated so far and was therefore the scope of this analysis.
Methods: For this purpose we performed amplicon-based sequencing (TruSight Myeloid 54-gene panel, Illumina®) of pre-transplant samples from 128 patients with MDS (85%) or sAML (15%) who had received an allo-SCT at our institution between 2001 and 2015 from related (46%) or unrelated (54%) donors following standard-dose (39%) or reduced-intensity (61%) conditioning. Seventy-three patients (57%) were directly transplanted (upfront group), while 55 patients (43%) had received pre-transplant cytoreductive therapy (CTX or HMA). Molecular data, pre-transplant therapy and clinical variables were entered into uni- and multivariate analyses to estimate their impact on outcome after allo-SCT and response to salvage therapy in patients relapsing after allo-SCT.
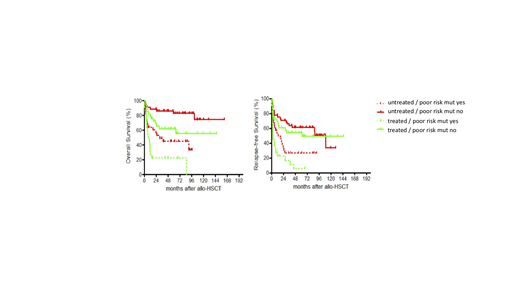
Results: Estimated 5-year overall (OS) and relapse-free survival (RFS) of the entire cohort was 56% and 42%, respectively. Corresponding to the presence of at least one mutation in 87% of patients (median 2 mutations per patient, range 0-6) a total of 285 mutations were detected, with RUNX1 (18%), ASXL1 (17%), SRSF2 (15%), DNMT3A (13%), TET2 (13%), TP53 (12%) and SF3B1 (10%) being the most frequently affected genes. Multivariate modeling revealed independent negative prognostic impact of TP53 and SF3B1 mutations on OS, RFS and cumulative incidence of relapse (CIR); NRAS and DNMT3A mutations on OS; as well as NRAS and SF3B1 mutations on non-relapse mortality. Similar to the non-transplant setting, these 4 "poor risk" mutations refined the prognostication of patients with complex karyotype, since those carrying at least one of the "poor-risk" mutations had an even worse prognosis. In addition, pre-transplant cytoreductive therapy negatively influenced not only OS, RFS and CIR after allo-SCT in multivariate analysis, but also interacted with mutations status. Indeed, patients receiving upfront Tx had the best outcome in terms of OS and RFS in the absence of "poor risk" mutations. In the presence of any of these 4 mutations, prognosis of patients receiving upfront TX was worse, but still significantly better compared to patients carrying a "poor risk" mutation who had undergone pre-transplant cytoreduction (Figure 1). The negative impact of these mutations detected prior to transplant was abrogated after transplant in 41 relapsed patients treated with HMA+/-DLI. Pre-transplant strategy also significantly influenced outcome after HMA-based salvage therapy, as patients in the upfront group had a higher likelihood of response and survival.
Conclusion:
These results confirm and expand previous data on the prognostic impact of mutations in TP53, SF3B1, DNMT3A and NRAS on outcome after allo-SCT. There seems be a prognostic interaction between mutational status and pre-transplant strategy, suggesting that it may be possible to adapt pre-transplant therapy according to the molecular profile of each patient.
Rautenberg:Jazz Pharmaceuticals: Other: Travel Support; Celgene: Honoraria, Other: Travel Support. Germing:Novartis: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria; Celgene: Honoraria, Research Funding; Amgen: Honoraria. Gattermann:Alexion: Research Funding; Novartis: Honoraria; Takeda: Research Funding. Kobbe:Celgene: Honoraria, Other: Travel support, Research Funding; Jazz: Honoraria, Other: Travel support; Medac: Honoraria, Other: Travel support; Roche: Honoraria, Other: Travel support; Amgen: Honoraria, Other: Travel support, Research Funding; Biotest: Honoraria, Other: Travel support; Novartis: Honoraria, Other: Travel support; Neovii: Honoraria, Other: Travel support; MSD: Honoraria, Other: Travel support; Pfizer: Honoraria, Other: Travel support; Takeda: Honoraria, Other: Travel support; Abbvie: Honoraria, Other: Travel support. Schroeder:Celgene Corporation: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal