Background: Sarcopenia, the loss of muscle mass, has been recognized as a prognostic factor for cancer patients. For example, low body mass index (BMI) was reported to be a risk of poor overall survival (OS) among allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients. However, low BMI was not associated with high non-relapse mortality (NRM) rate, and BMI may not directly reflect the physical condition. (Bone Marrow Transplant. 2014;49:1505-12). To evaluate the clinical impact of the muscle volume on the prognosis of allo-HSCT recipients, other biomarkers that directly reflect muscle mass may be warranted.
Urinary creatinine excretion (UCE) has been reported to estimate muscle mass and have prognostic value for kidney transplant patients (Transplantation. 2008;86:391-8.). There is no report to evaluate clinical impact of UCE on the prognosis of allo-HSCT recipients. Therefore, we retrospectively analyzed the association between pre-transplant UCE and the transplant outcomes.
Methods: We included 173 adult patients with acute myeloid leukemia (AML) in complete remission (CR) who underwent first allo-HSCT from 2006 to 2017 at our institute and measured UCE before allo-HSCT. Concerned the possibility of urine storage failure, two patients with low total daily urine volume (<0.5L/day) were excluded from this analysis. Therefore, we investigated the remaining 171 patients. In order to correct the physical disparities of individual patients, we evaluated the clinical impact of weight adjusted UCE (WA-UCE) ,i.e UCE / body weight [μmol/kg/day] (Intensive Care Med. 2018;44:1699-708.).
We used receiver operating characteristics curve in order to determine the cutoff value of the WA-UCE and classified the patients into the high and low WA-UCE group. We evaluated transplant outcomes such as OS, progression-free survival (PFS), NRM, and cumulative incidence of relapse (CIR) between two groups.
Results: The median age at allo-HSCT was 52 (range, 18-73) and there were more male patients (n=111) than female patients (n=60). Regarding cytogenetic risk, 15 (9.1%), 112 (65.8%), and 38 (23.0%) were categorized as favorable, intermediate, and poor risk, respectively (There were five patients without cytogenetic data). The median follow-up period of survivors was 704 (range, 9 to 3,857) days.
We defined the cutoff value of the weight adjusted UCE as 148 μmol/kg/day in male and 128 μmol/kg/day in female. Among 171 patients, 90 patients (male = 59, female = 31) were in the high WA-UCE group and 81 patients (male = 52, female = 29) were in the low WA-UCE group. We found no significant differences between two groups in terms of the number of relapse before allo-HSCT, cytogenetic risks, conditioning regimens, hematopoietic cell transplantation comorbidity index, donor-recipient HLA matching, donor source, or estimated glomerular filtration rate. On the other hand, patient's age at allo-HSCT was significantly younger (low vs. high WA-UCE group: median, 53 [range, 18 - 73] vs. 48 [range, 19 - 68] years, P = 0.02) and BMI was lower (low vs. high WA-UCE group: median, 22.3 [range, 15.4 - 38.8] vs. 21.9 [range, 15.4 - 29.3] kg/m2, P = 0.003) in high WA-UCE group.
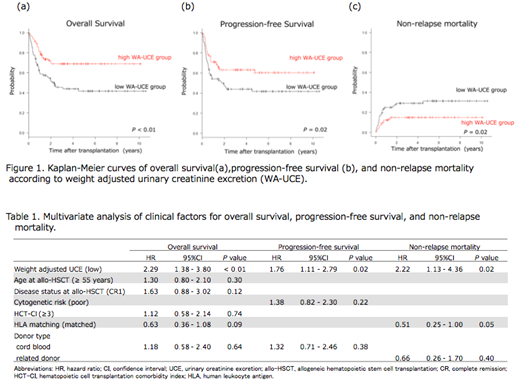
In univariate analysis, we observed a significant difference in OS, PFS, and NRM between two groups (low vs. high WA-UCE group: 1-year OS, 60.1% vs. 80.9%, P < 0.01; 1-year PFS, 54.1% vs. 70.9%, P = 0.02; 1-year NRM, 24.8% vs. 12.3%, P = 0.02) (Figure1). On the other hand, there was no significant difference in 1-year CIR between two groups (low vs. high WA-UCE group: 21.1% vs. 16.8%, P = 0.63). In our cohort, the low BMI (< 18.5 kg/m2) was not significantly associated with OS, PFS, CIR, and NRM (low vs. high BMI group: 1-year OS, 77.6% vs. 69.9%, P = 0.51; 1-year PFS, 74.1% vs. 60.9%, P = 0.45; 1-year CIR, 14.8% vs. 19.5%, P = 0.02, 1-year NRM, 11.1% vs. 19.5%, P = 0.70)
In multivariate analysis, the low WA-UCE was an independent risk factor for OS (Hazard ratio (HR) [95% confidence interval (CI)]; 2.29 [1.38 - 3.80], P < 0.01), PFS (HR [95% CI]; 1.76 [1.11 - 2.79], P = 0.02), and NRM (HR [95% CI]; 2.22 [1.13 - 4.36], P = 0.02) (table1).
Conclusion: In allo-HSCT adult recipients with AML in CR, low WA-UCE before transplantation was associated with poor prognosis, which related to high NRM within 1 year. WA-UCE can be an independent, objective, simple, and reliable biomarker for evaluating muscle mass and predicting transplant outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal