
Introduction: Selection of an appropriate donor is important for the success of allogeneic hematopoietic stem cell transplantation (allo-HCT). In general, an HLA-matched related donor (M-RD) and an HLA-matched unrelated donor (M-UD) are considered to be the first and second preferred donors in allo-HCT. On the other hand, the most suitable alternative donor remains unclear, when M-RD and M-UD are unavailable. In addition, the information on a suitable donor selection for elderly patients are limited. We hypothesized that the patient age might change the donor selection priority in allo-HCT, because related donor age, organ function, or endurance against graft-versus-host disease or infection differ between younger and older patients. Therefore, we conducted a nationwide, large retrospective study to investigate the donor selection priority in allo-HCT, classified according to patient age.
Methods: We analyzed 17848 adult patients with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome who underwent a first all-HCT between 2007 and 2017 in Japan. We compared the transplant outcomes among M-RD (n=4106), HLA 1-antigen-mismatched related donor (1MM-RD) (n=592), HLA 2,3-antigen-mismatched related donor (23MM-RD) (n=882), M-UD (n=3927), HLA 1-locus-mismatched unrelated donor (1MM-UD) (n=2474), and unrelated cord blood (U-CB) (n=5867), in the whole cohort and in subgroups of patients aged <50 years (n=8572) and those aged ≥50 years (n=9275). All P-values were two sided, and P-values <0.05 were considered statistically significant.
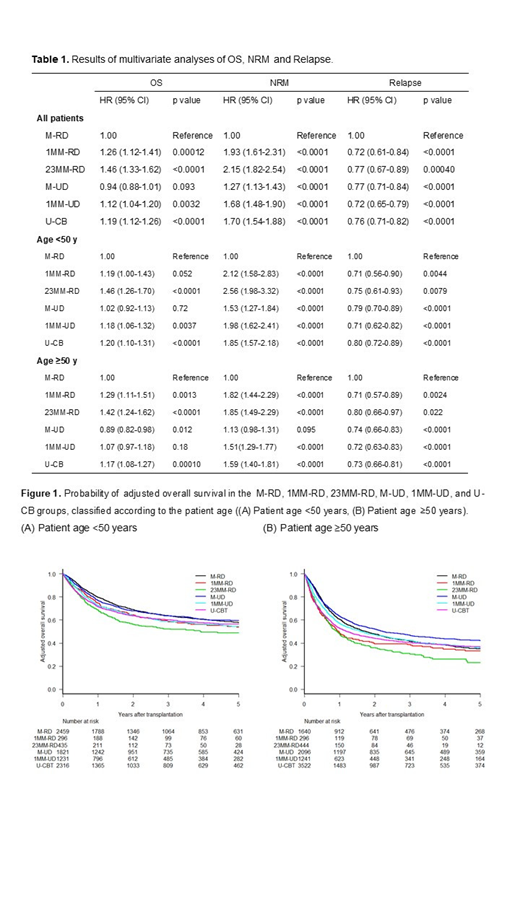
Results: Results of multivariate analyses of overall survival (OS), non-relapse mortality (NRM) and relapse are summarized in Table 1. For all patients, 1MM-RD, 23MM-RD, 1MM-UD, and U-CB were independent significant adverse factors for OS and NRM (hazard ratio [HR] 1.26, p<0.001 and HR 1.93, p<0.001 for 1MM-RD; HR 1.46, p<0.001 and HR 2.15, p<0.001 for 23MM-RD; HR 1.12, p=0.0032 and HR 1.68, p<0.001 for 1MM-UD; and HR 1.19, p<0.001 and HR 1.70, p<0.001 for U-CB) compared to M-RD. On the other hand, there was no significant difference in OS between the M-RD and M-UD groups (HR 0.94, p=0.093), although NRM in the M-UD was inferior to that in the M-RD group (HR 1.27, p<0.001). The risk of relapse in the 1MM-RD, 23MMRD, M-UD, 1MM-UD, and U-CB groups were significantly lower than that in the M-RD group. An interaction test revealed that the effect of M-UD on OS and NRM differed between the patient age <50 years and ≥50 years (HR 0.88, p=0.055 and HR 0.78, p=0.019). Next, we compared transplant outcomes among these donor source within subgroups of patients grouped according to patient age. For the patients aged <50 years, 23MM-RD, 1MM-UD, and U-CB were independent significant adverse factors for OS (HR 1.46, p<0.001, HR 1.18, p<0.001 and HR 1.20, p<0.001), and 1MM-RD tended to be an adverse factor for OS (HR 1.19, p=0.052). On the other hand, OS in the M-UD group was comparable to that in the M-RD group (HR 1.02, p=0.72). For the patients aged ≥50 years, 1MM-RD, 23MMRD, and U-CB were independent significant adverse factors for OS (HR 1.29, p=0.0013, HR 1.42, p<0.001 and HR 1.17, p<0.001). 1MM-UD was not a significant adverse factor for OS (HR 1.07, p=0.18). Conversely, OS in the M-UD group was superior to that in the M-RD group (HR 0.89, p=0.012). In addition, we classified the M-UD group according to donor age (M-UD-Y, donor age <50 years; M-UD-O, donor age ≥50 years). For the patients aged ≥50 years, OS in the M-UD-Y group was superior to that in the M-RD group (HR 0.86, p=0.035), although there was no significant difference in OS between the M-UD-O and M-RD groups (HR 1.08, p=0.70). However, for the patients aged <50 years, OS in the both M-UD-Y and M-UD-O groups were comparable to those in the M-RD group (HR 1.02, p=0.73 and HR 1.04, p=0.82). The probabilities of adjusted OS at 3 years for the patients aged <50 years and aged ≥50 years were 63.6% and 41.6% in the M-RD group, 58.4% and 38.8% in the 1MM-RD group, 52.3% and 30.4% in the 23MM-RD group, 63.6% and 46.8% in the M-UD group, 58.9% and 42.3% in the 1MM-UD group, and 60.1% and 40.3% in the U-CB group (Figure 1).
Conclusions: Donor selection priority in all-HCT might be different according to patient age. In particular, young M-UD might be the first preferred donor for patients aged 50 years or older. We should reconsider donor selection priority in allo-HCT based on patient and donor age.
Kanda:Takeda: Honoraria; Celgene: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Chugai: Honoraria; Kyowa Hakko Kirin: Honoraria; Otsuka: Honoraria; Bristol-Meyers Squib: Honoraria; JCR Pharmaceuticals: Honoraria; MSD: Honoraria; Daiichi Sankyo Company: Honoraria; NextGeM Incorporation: Patents & Royalties: 2019-011392. Kimura:JSPS KAKENHI: Research Funding. Ozawa:Novartis: Honoraria; Pfizer Japan Inc.: Honoraria; Astellas Pharma Inc.: Honoraria; Kyowa-Hakko Kirin: Honoraria. Atsuta:Kyowa Kirin Co., Ltd: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria. Kanda:Pfizer: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Asahi-Kasei: Research Funding; CSL Behring: Research Funding; MSD: Research Funding; Pfizer: Research Funding; MSD: Research Funding; Ono: Consultancy, Honoraria, Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Tanabe Mitsubishi: Research Funding; CSL Behring: Research Funding; Novartis: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Asahi-Kasei: Research Funding; Otsuka: Research Funding; Tanabe Mitsubishi: Research Funding; Dainippon Sumitomo: Consultancy, Honoraria, Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Taisho-Toyama: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Taiho: Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Celgene: Consultancy, Research Funding; Dainippon Sumitomo: Consultancy, Honoraria, Research Funding; Mochida: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Sanofi: Research Funding; Takara-bio: Consultancy, Honoraria; Taisho-Toyama: Research Funding; Taiho: Research Funding; Nippon-Shinyaku: Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Mochida: Consultancy, Honoraria; Celgene: Consultancy, Research Funding; Takara-bio: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Nippon-Shinyaku: Research Funding; Ono: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal