[Background] We previously demonstrated that allogeneic hematopoietic stem cell transplantation (HSCT) in the first complete remission (CR1) was recommended for adult patients wih Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) who have a human leukocyte antigen (HLA)-matched related (Leukemia 2011;25:259-265) or unrelated donor (Bone Marrow Transplant 2013;48:1077-1083). However, pediatric-inspired high-intensity chemotherapy dramatically improved the prognosis of adult patients with Ph-negative ALL. Therefore, the optimal treatment strategy for Ph-negative ALL in CR1 has not been established in the era of high-intensity chemotherapy.
[Methods] Outcomes of patients with Ph-negative ALL who underwent HSCT from HLA-matched related or unrelated donor in CR1 performed between 2002 and 2011 (HSCT-MRD group and HSCT-MUD group, respectively) were obtained from the Transplant Registry Unified Management Program (TRUMP), which is the registry database of the Japanese Society for Hematopoietic Cell Transplantation (JSHCT). Patients aged between 16 and 24 and between 25 and 65 were analyzed separately, and their outcomes were compared with those of patients who did not receive HSCT in CR1 in clinical studies by Japan Adult Leukemia Study Group (JALSG), ALL-202U study in which patients received chemotherapy designed for pediatric patients (Blood Cancer J 2014;4:e252)(202U group) and ALL-202O study in which patients were randomly assigned to receive adult-type chemotherapy with high-dose or intermediate-dose methotrexate (MTX) therapy (Leukemia 2018;32:626-632)(202O group), respectively. Median durations between diagnosis to HSCT were obtained from the TRUMP data, and only patients who were disease-free more than each median duration were included from the JALSG studies. Risk stratifications were performed based on the white blood cell (WBC) count and cytogenetics.
[Results]
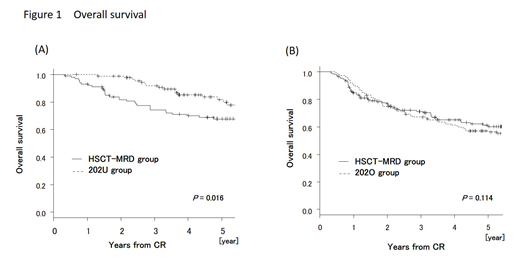
In patients aged less than 25 who underwent HSCT from related donor, the median duration between diagnosis to HSCT was 188 days. Overall survival (OS) in 202U group (N = 93) was significantly superior compared with OS in HSCT-MRD group (N = 102) (OS at 5 years: 81.8% vs 67.8%, P = 0.016) (Figure 1(A)), and which was attributed to the higher non-relapse mortality in HSCT-MRD group (NRM at 5 years: 1.4% vs 9.5%, P = 0.015). Disease-free survival (DFS) and relapse rate (RR) were similar between two groups (DFS at 5 years: 70.2% vs 62.5%, P = 0.115, and RR at 5 years: 28.4% vs 28.4%, P = 0.689). Higher OS was preserved in younger (<21) and standard-risk patients. In patients aged less than 25 who underwent HSCT from unrelated donor, the median duration between diagnosis to HSCT was 246 days. OS in 202U group (N = 92) was similar with OS in HSCT-MUD group (N = 51) (OS at 5 years: 81.6% vs 82.7%, P = 0.772). RR in HSCT-MUD group was lower (RR at 5 years: 16.7%) than that in HSCT-MRD group.
In patients aged more than 24 who underwent HSCT from related donor, the median duration between diagnosis to HSCT was 158 days. OS rate was not different between 202O group (N = 147) and HSCT-MRD group (N = 152) (OS at 5 years: 57.0% vs 60.2%, P = 0.114) (Figure 1(B)). The lower RR was counterbalanced by the higher NRM in HSCT-MRD group (RR at 5 years: 51.6% vs 30.0%, P < 0.001, and NRM at 5 years: 4.1% vs 15.2%, P = 0.015). No difference in OS was demonstrated among any subgroups. Superior DFS in HSCT-MRD group was observed only in high-risk patients (DFS at 5 years: 26.5% vs 57.2%, P = 0.003), but it diminished when only patients who actually received high-dose MTX were included in 202O group (DFS at 5 years: 36.4% vs 57.2%, P = 0.092). In patients aged more than 24 who underwent HSCT from unrelated donor, the median duration between diagnosis to HSCT was 214 days. OS rate was not different between 202O group (N = 138) and HSCT-MUD group (N = 104) (OS at 5 years: 60.0% vs 65.8%, P = 0.563). Superior DFS in HSCT-MUD group was observed even in all patients (DFS at 5 years: 47.2% vs 64.2%, P = 0.029). RR in HSCT-MUD group was lower (RR at 5 years: 14.3%) than that in HSCT-MRD group.
[Discussions & Conclusions] High-intensity chemotherapy may change the role of HSCT for Ph-nagative ALL. Improved outcomes in HSCT-MUD group were observed, and it was partially because patients who had long CR before HSCT could underwent HSCT from MUD in CR1.
Kako:Bristol-Myers Squibb: Honoraria; Pfizer Japan Inc.: Honoraria. Tanaka:Bristol-Myers Squibb: Research Funding. Ichinohe:JCR Pharmaceuticals: Honoraria; Celgene: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Astellas Pharma: Research Funding; Chugai Pharmaceutical Co.: Research Funding; CSL Behring: Research Funding; Eisai Co.: Research Funding; Kyowa Hakko Kirin Co.: Research Funding; Ono Pharmaceutical Co.: Research Funding; Pfizer: Research Funding; Nippon Shinyaku Co.: Research Funding; MSD: Research Funding; Otsuka Pharmaceutical Co.: Research Funding; Repertoire Genesis Inc.: Research Funding; Sumitomo Dainippon Pharma Co.: Research Funding; Taiho Pharmaceutical Co.: Research Funding; Takeda Pharmaceutical Co.: Research Funding; Zenyaku Kogyo Co.: Research Funding; Alexion Pharmaceuticals: Honoraria; Bristol-Myers Squibb: Honoraria; Mundipharma: Honoraria; Novartis: Honoraria. Kurahashi:Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Novartis Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd: Honoraria; Bristol-Myers Squibb, Ltd.: Honoraria. Usui:Alexion Pharmaceuticals: Other: Personal Fees; Bristol-Myers Squibb: Other: Personal Fees, Research Funding; Kyowa-Hakko-Kirin Co.Ltd.: Other: Personal Fees; Astellas Pharma Inc.: Other: Personal Fees; Huya Bioscience International: Other: Personal Fees; Nippon Shinyaku Co.: Other: Personal Fees; SymBio Pharmaceuticals: Other: Personal Fees; Celgene Corporation: Other: Personal Fees; Pfizer: Other: Personal Fees, Research Funding; Takeda Pharmaceutical Co. Ltd: Other: Personal Fees; Cmic Co: Other: Personal Fees; Otsuka Pharmaceutical Co.Ltd.: Other: Personal Fees; AbbVie Inc: Other: Personal Fees. Kiyoi:Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Astellas Pharma Inc.: Honoraria, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Pfizer Japan Inc.: Honoraria; Perseus Proteomics Inc.: Research Funding; Otsuka Pharmaceutical Co.,Ltd.: Research Funding; Bristol-Myers Squibb: Research Funding; Daiichi Sankyo Co., Ltd: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; FUJIFILM Corporation: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding. Matsumura:Bristol-Myers Squibb: Speakers Bureau; Novartis: Speakers Bureau; Otsuka Pharmaceutical: Consultancy, Research Funding; Pfizer: Research Funding, Speakers Bureau. Miyazaki:Chugai: Research Funding; Otsuka: Honoraria; Novartis: Honoraria; Nippon-Shinyaku: Honoraria; Dainippon-Sumitomo: Honoraria; Kyowa-Kirin: Honoraria. Atsuta:CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal