BACKGROUND
The efficacy of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in acute myeloid leukemia (AML) relies on the antileukemic effect of the conditioning regimen and the graft-versus leukemia (GVL) effect mediated by donor T-cells. Leveraging the GVL effect while reducing toxicity enables reduced intensity conditioning (RIC) to be used to older or frail patients, but may lead to higher rates of graft-versus-host disease (GVHD), impairing later quality of life. Novel T-cell modulation strategies may limit the incidence of GVHD while preserving antileukemic activity leading to better outcomes.
Herein, we compare the outcomes of AML patients treated with myeloablative (MAC) and RIC regimens including patients receiving dual T-cell depletion using Anti-Thymocyte Globulin (ATG) and Post-Transplant Cyclophosphamide (PTCy).
METHODS
Between 2013 and 2018, 362 adults with AML in complete remission underwent allo-HSCT and were included in the study.
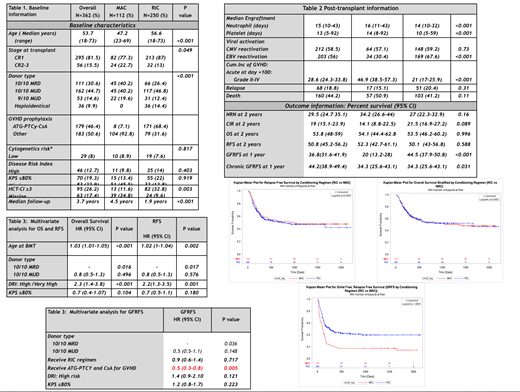
Overall, 112 (31%) patients received MAC and 250 (69%) received RIC regimens. MAC consisted of Flu(4), Bu (4mg) and 400cGy of total body irradiation (TBI). RIC regimen consisted on Flu (4), Bu (2) and 200cGy TBI in 249 recipients and one received clofarabine/busulfan. One hundred seventy-one (68.4%) the recipients who received RIC regimen, received a novel dual T-cell depletion strategy using ATG (total dose of 4.5mg/kg in 130 patients and 2mg/kg in 41 recipients), PTCy, and cyclosporine for GVHD prophylaxis. Peripheral blood stem cell (PBSC) grafts were used in 98.9% of the recipients.
Data was collected retrospectively and updated on April 2019. The cumulative incidence of GVHD was assessed accounting relapse and death as competing events. A multivariate analysis for overall survival (OS), relapse-free survival (RFS) and GVHD-Free/RFS (GFRFS) was conducted controlled by those variables found to be significant in univariate analysis.
RESULTS
Baseline information is summarized in Table 1. The incidence of grade II-IV and III-IV acute GVHD at day +100, and moderate/severe chronic GVHD at 1 year was respectively: 28.6%, 10.5%, and 18.4%. RIC was associated with a significantly lower cumulative incidence of clinically relevant acute and chronic GVHD (P<0.05).
Main outcome information is summarized in tables 1 and 2, and displayed graphically in figures A-F. With a median follow-up of 3.7 years, 68 (18.8%) recipients relapsed and 160 (44.2%) died. The 2-years OS, RFS, and GRFS were 53.8%, 50.8%, and 33.8%, respectively. OS, RFS, non-relapse mortality, and cumulative incidence of relapse were not significantly affected by regimen intensity (P>0.05). However, RIC regimens were associated with superior GRFS.
Forty-three (12.7%) recipients had a high Disease Risk Index (DRI). Conditioning intensity was not a significant risk factor for OS (HR 0.9; P=0.931) or RFS (HR 1; P=0.902) in this group of patients.
Tables 3 and 4 show the results from multivariate analysis for OS, RFS, and GFRFS. Older patients, those with high DRI, or recipients of 9/10 matched unrelated donor (MUD) grafts had significantly worse OS and RFS.
GVHD prophylaxis with dual T-cell modulation (ATG-PTCy-CsA) was the only protective parameter for a better GRFS (HR 0.5; P=0.005).
CONCLUSION
In AML patients, RIC with PBSC allografts and dual T-cell modulation with ATG and PTCy led to superior GRFS when compared with MAC regimens. Reduction in the cumulative incidence of clinically significant acute and chronic GVHD may be possible without compromising on the efficacy of the GVL effect. The use of this GVHD prophylaxis strategy, along with mitigation of conditioning toxicity by using RIC, may result in better quality of life.
Further investigations would be done with the use of this novel GVHD prophylaxis in the setting of MAC regiments.
Dual T-cell suppression leads to increased infectious complications including viral reactivations. The use of lower doses of ATG, or individualized dosing strategies based on lymphocyte count may help to further optimize this strategy.
Michelis:CSL Behring: Other: Financial Support. Mattsson:Celgene: Honoraria; Therakos: Honoraria; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal