Introduction: AML patients with intermediate or high-risk features often undergo allogeneic hematopoietic cell transplantation (alloHCT) during first complete remission (CR). The 2017 European LeukemiaNet guidelines for AML specify categories of CR: both with and without count recovery (CR vs. CRi) and with and without measurable residual disease (MRD). Previous smaller retrospective studies have suggested poorer survival outcomes after alloHCT for patients with responses less than CR.
Methods: Eligible cases were determined using the CIBMTR registry. Each had AML in CR1, was ≥ 18 years, and underwent alloHCT between January 1, 2007 and December 31, 2015. MRD was defined based on the answers to qualitative questions on standard clinical reporting forms that ask if the patient is in either molecular or cytogenetic remission and if disease is detected in marrow by flow cytometry at time of HCT. The primary outcome was overall survival (OS), and secondary outcomes were non-relapse mortality (NRM), relapse and disease-free survival (DFS). The Kaplan-Meier method was used to estimate survival and cumulative incidence function was used to estimate relapse and NRM. Multivariable analysis (MVA) was performed using the Cox proportional hazards model to adjust for patient-, disease-, and transplant-related factors. Adjusted probabilities of DFS and OS, adjusted cumulative incidence curves of NRM and relapse were generated from final Cox regression models stratified on CR vs. CRi and weighted averages of covariate values using pooled sample proportion as weight function.
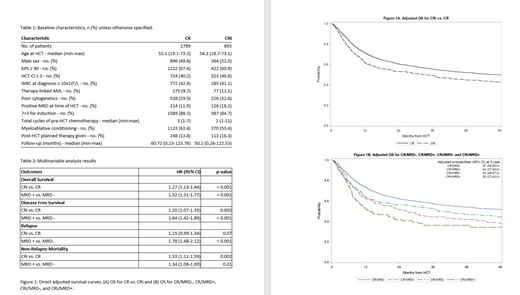
Results: We identified 2492 cases (CR, n=1799; CRi, n=693). The main effect variable (CR vs. CRi) was missing in 262 additional patients; these patients were excluded when univariate analysis confirmed no significant associations, suggesting a random distribution of missing data. Patient characteristics are summarized in Table 1. Compared with patients with CR, patients with CRi were more likely to have a Karnofsky score <90 (38% vs. 31%) and an HCT-CI score 3+ (47% vs. 40%). Other variables were well matched between the groups. MVA demonstrated significantly increased likelihood of mortality in patients with CRi compared to those with CR with hazard ratio (HR) 1.27; 95% confidence interval (CI) (1.13-1.43) (Figure 1A). Other covariates significantly associated with shorter OS included older age, poor-risk cytogenetics, lower Karnofsky score, higher HCT-CI score, and higher white blood cell count at diagnosis. The adjusted OS probabilities at 5 year post-HCT accounted for factors from MVA model are 50% (95%CI 47-52) for patients with CR and 43% (95%CI 39-47) for patients with CRi. CRi was also associated with significantly increased NRM [HR 1.33, 95%CI(1.11-1.59)] with only a trend in increased relapse [HR 1.15, 95% CI(0.99-1.34), p=0.07] resulting in inferior DFS [HR 1.20, 95%CI(1.07-1.35)]. MRD status was available in a subset of 2297 patients, and pairwise comparison demonstrated that presence of MRD was associated with shorter OS, shorter DFS, higher NRM, and increased relapse compared to absence of MRD (Figure 1B). Pairwise interaction between the main effects (CR vs. CRi) and MRD status were tested with no significant findings at a level of 0.01, demonstrating that the effects of incomplete count recovery and MRD are independent of each other.
Conclusions: Analysis of this large CIBMTR cohort demonstrates that survival outcomes differ among AML patients nominally in CR at the time of alloHCT. Patients with CRi and/or MRD have significantly shorter OS after alloHCT compared to those in CR, as well as shorter DFS and higher NRM. Further studies should focus on limiting NRM and reducing relapse to optimize post-alloHCT outcomes for patients with responses less than CR.
Percival:Pfizer Inc.: Research Funding; Nohla Therapeutics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Kebriaei:Jazz: Consultancy; Pfizer: Honoraria; Amgen: Research Funding; Kite: Honoraria. Weisdorf:Fate Therapeutics: Consultancy; Incyte: Research Funding; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal