Engraftment syndrome (ES) is a well-defined entity characterized by non-infectious fever and other clinical manifestations including skin rash, pulmonary infiltrates, diarrhea, weight gain and neurological symptoms which happens in the setting of autologous HSCT during early neutrophil recovery phase. (Spitzer ,2001).These clinical manifestations usually occur immediately before or at the time of neutrophil engraftment possibly due to the release of inflammatory cytokines. ES may require therapy with corticosteroids and other immunosuppressive drugs.
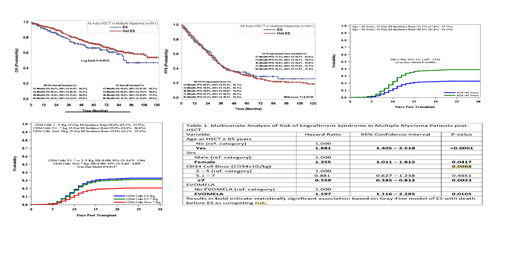
Our study cohort included 645 patients with multiple myeloma treated with autologous stem cell transplantation between January 2010 and June 2019. The majority of patients had a single autologous transplant (80%), 18 % received a second autologous transplant and 3 patients had a third autologous transplant. Fifty seven percent of patients were male, 61 % had IgG myeloma and 50 percent had standard risk cytogenetics. Sixty three percent of patients were under the age of 65 years. ES was defined as a combination of at least 2 symptoms not attributed to other causes, including non-infectious fever, diarrhea, skin rash, pulmonary infiltrates or hepatic dysfunction, occurring from 3 days prior to 10 days post engraftment. (Cornell ,2015).One hundred and ninety seven patients in this cohort met the criteria for engraftment syndrome of whom 173 were treated with corticosteroids and 9 required the addition of tacrolimus or cyclosporine. Univariate and multivariate statistical analyses were performed looking at risk factors for the development of ES and the overall effect of ES on patient outcome.
Results of our univariate analysis showed that age >65, female sex, use of plerixafor were significant risk factors for developing engraftment syndrome while use of cyclophosphamide-based mobilization had significantly reduced risk. Multivariate analysis using Gray Fine model revealed that patients over 65 years were twice as likely to develop ES than patients who were younger than 65 years (HR=1.881, CI: 1.405 to 2.518). Females had a 36% higher risk of ES than male patients (HR=1.355, CI: 1.011 to 1.815). Patients who were infused with more than 7x106 CD34+ cells/kg had a 40% reduced risk of developing ES (HR=0.559, CI: 0.385 to 0.812). Receiving the new formulation of melphalan: EVOMELAⓇ, as preparative regimen, was associated with a 60% increased risk of developing ES compared to patients treated with the standard formulation (HR=1.597, CI: 1.116 to 2.285). The use of plerixafor was found to be a risk factor for ES even when adjusted for age(HR=1.463,CI:1.024 to 2.089).
Follow- up of patients that did not develop ES (n=445) had a median of 59 months (IQR: 29.0 -80.0months), range: 0 - 136 months. Follow-up time of patients that developed ES (n=197) was 41.0 months (IQR: 16.0 - 66.0 months), range: 0.0 - 131 months.An overall survival analysis of patients who developed engraftment syndrome showed a trend for improved survival in patients who did not develop engraftment syndrome, however this did not meet statistical significance and PFS curves were similar with no statistically significant difference between the two groups.
Our study of this large cohort of patients suggests that selection of mobilization regimen and conditioning chemotherapy could decrease the incidence of ES, thereby decreasing morbidity and prolonged hospital stay. There can also be a consideration for pre-emptive treatment of patients in the very high risk category based on age, gender and available cell dose.
Siegel:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Rowley:Allergan: Equity Ownership; Fate Therapeutics: Consultancy. Biran:Amgen: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol Meyers Squibb: Research Funding. Goldberg:Cancer Outcomes Tracking and Analysis (COTA) Inc.: Equity Ownership; Bristol-Myers Squibb: Consultancy; COTA: Equity Ownership. Goy:Takeda: Other: Grants outside of the submitted work; Hackensack University Medical Center, RCCA: Employment; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Hakensackumc: Research Funding; University of Nebraska: Research Funding; Astrazenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Other: Grants outside of the submitted work, Research Funding; Pharmacyclics/Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: leadership role for profit healthcare company; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal