Introduction: Chronic graft-versus-host disease (cGVHD) is a serious complication of allogeneic hematopoietic stem cell transplantation. Pulmonary cGVHD consists of both obstructive and restrictive pulmonary changes, and as such, represents a broad spectrum of disease. While corticosteroids are a mainstay of treatment, there is no established standard-of-care for second line therapy. The Bruton's tyrosine kinase inhibitor ibrutinib was recently approved for treatment of cGVHD after failure of 1 or more lines of systemic therapy. However, the role of ibrutinib in patients with pulmonary cGVHD is not well understood. Here we report an institutional experience of ibrutinib as non-first-line therapy for pulmonary cGVHD.
Methods: We performed a retrospective study of adult patients with steroid-dependent or steroid-refractory pulmonary cGVHD treated with ibrutinib as non-first line therapy between 2014 and 2018. Patients were diagnosed with pulmonary cGVHD if they met the 2014 National Institutes of Health cGVHD Consensus Panel criteria or were diagnosed based on expert opinion. All patients received appropriate GVHD prophylaxis at the time of transplant. The severity of pulmonary cGVHD was assessed by pulmonary function testing (PFT) done prior to and 180 days after initiation of ibrutinib therapy. The primary clinical outcome was absolute change in the percentage of predicted forced expiratory volume in 1 second (%FEV1) over 180 days. Patients were identified as having a complete response (normalization of %FEV1), partial response (≥10% increase in %FEV1), stable disease (<10% change in %FEV1), or progression (≥10% decrease in %FEV1).
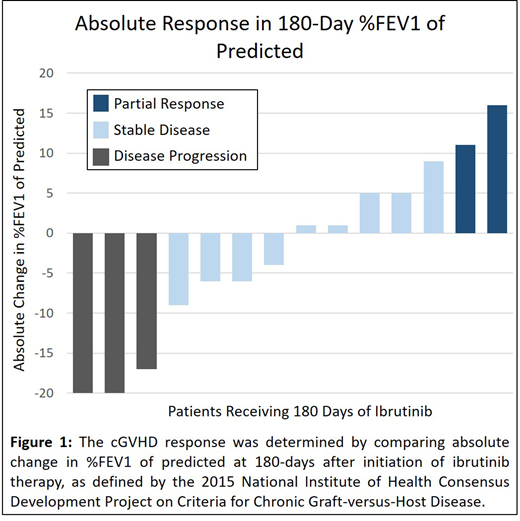
Results: A total of 17 patients received ibrutinib for pulmonary cGVHD (mean age 46 ± 11 years, 59% male), with a mean duration of 34 ± 17 weeks from transplant to diagnosis of cGVHD (31% de novo, 31% progressive, 38% interrupted). On average, patients had 5 involved organs, received 4 treatments for cGVHD prior to ibrutinib, and 14/17 (82%) were on at least 0.25 mg/kg/day of prednisone (or an equivalent dose of corticosteroid) at the time of ibrutinib initiation. The median %FEV1 at time of ibrutinib initiation was 50 ± 24%. At 180 days after initiation, 14/17 patients (82%) continued receiving ibrutinib (1 patient each discontinued therapy for lack of symptomatic improvement, oral ulcers, and enrollment in a clinical trial). Among the 14 patients who completed 180 days of therapy, only 3 had disease progression (Figure 1).
Conclusions: Ibrutinib was generally well tolerated as a non-first line therapy for pulmonary cGVHD. In this cohort of patients with steroid-dependent or steroid-refractory disease who had progression on multiple lines of therapy, most had stable or improved pulmonary function by %FEV1 while on ibrutinib. Given the heterogeneity of pulmonary cGVHD, further studies can help clarify which patients are most likely to demonstrate response.
Miklos:Becton Dickinson: Consultancy; Adaptive Biotechnologies: Consultancy, Research Funding; Pharmacyclics: Consultancy, Patents & Royalties, Research Funding; Celgene-Juno: Consultancy; AlloGene: Consultancy; Janssen: Consultancy; Precision Bioscience: Consultancy; Kite, A Gilead Company: Consultancy, Research Funding; Miltenyi: Consultancy, Research Funding; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal