Background : The conditioning regimen for patients with myelolfibrosis undergoing an allogeneic HSCT is usually composed of a combination of fludarabine (FLU) with one alkylating agent, busulkfan (BU), thiotepa (THIO) or melphalan. In a recent prospective randomized study comparing BU-FLU versus THIO-FLU, the proportion of patients with full donor chimerism at 6 months, was respectively 63% and 65% (Patriarca et al, BBMT 2019).
Aim of the study. Assess the rate of full donor chimerism in patients with myelofibrosis, after conditioning with one or two alkylating agents.
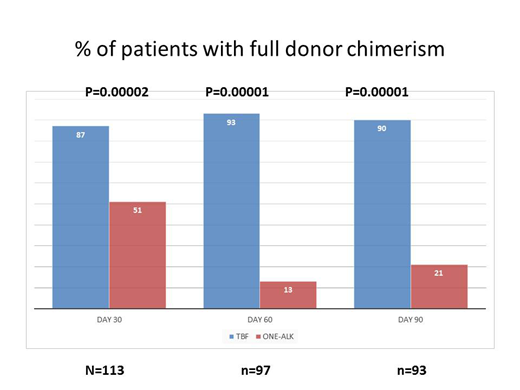
Methods. We analyzed 113 patients with myelofibrosis, for whom chimerism data were available on day +30 . There were two groups: 35 patients were conditioned with either thiotepa-cyclophosphamide , thiotepa-fludarabine or busulfan-fludarabine (ONE-ALK), whereas 78 patients were prepared with thiotepa, busulfan, fludarabine (TBF). Patients receiving TBF were older (57 vs 52 years, p=0.008), were less frequently splenectomized pre-HSCT (30% vs 54%, p=0.03), had more frequently intermediate-2/high DIPSS scores (89% vs 74%, p=0.04) , and had comparable transfusion burden pre-HSCT (p=0.7). Chimerism was assessed via STR (PowerFlex-Promega)
Results.
The proportion of patients with full donor chimerism on day +30 in the TBF vs the ONE-ALK group was 87% vs 51% (p=0.00002); on day +60 these figures were 93% vs 13% (p<0.0001) and on day +90 , the figures were 90% vs 21% (p<0.00001). Full donor chimerism on day+30 was achieved in 81% of patients with DIPSS int1 (n=16), 74% of patients with int2 DIPSS, and 70% of patients with high risk DIPSS (p=0.6).
Acute GvHD grade II-IV occurred in 27% vs 37% of patients in the two groups (p=0.7), and moderate severe chronic GvHD in 20% and 21% (p=0.8). The 5 year cumulative incidence of relapse was 8% in the TBF group, versus 50% for the ONE-ALK group (p<0.0001), whereas the CI of TRM was 25% vs 11% (p=0.1). The 5 year actuarial disease free survival (DFS) was respectively 65% for TBF and 38% for the ONE-ALK group (p=0.004).
Complete chimerism day+30. When looking at whether patients had (n=84) or not (n=29) full donor chimerism on day +30, the CI of relapse was respectively 44% vs 15% (p=0.002), the CI of TRM 18% vs 15% (p=0.5), and the 5 year DFS 65% vs 32% (p=0.001).
Conclusions. Early full donor chimerism is a prerequisite for long term control of disease in patients with myelofibrosis undergoing an allogeneic HSCT. The combination of 2 alkylating agents in the conditioning regimen, provides a significantly higher chance of achieving full donor chimerism on day+30, and thus long term disease control.
Angelucci:Vertex Pharmaceuticals Incorporated, and CRISPR Therapeutics: Other: Partecipation in DMC; Roche: Other: Local advisory board; Jazz Pharmaceuticals: Other: Local advisory board; BlueBirdBio: Other: Local advisory board; Novartis: Honoraria, Other: Chair Steering Committee TELESTO protocol; Celgene: Honoraria, Other: Partecipation in DMC.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal