The recent success of immunotherapy using chimeric antigen receptor modified T cells (CAR T) in B-cell malignancies highlights the potential of these cytotoxic "drugs" for cancer therapy. CAR T therapies generally rely upon manufacturing approaches that include prior T cell activation through engagement of the TCR and costimulatory receptors followed by ex vivo expansion of patient-derived T cells over days to weeks. We previously reported that CD3/CD28 stimulation prior to transduction promotes progressive T cell effector differentiation over time in culture with loss of CAR T cell potency (Ghassemi et al. 2018 PMID: 30030295). Since cell division is not a prerequisite for lentiviral vector-mediated gene delivery, we hypothesized that lentiviral transduction of quiescent T cells without prior activation will enhance engraftment and persistence of CART cells that is associated with long-term leukemia control.
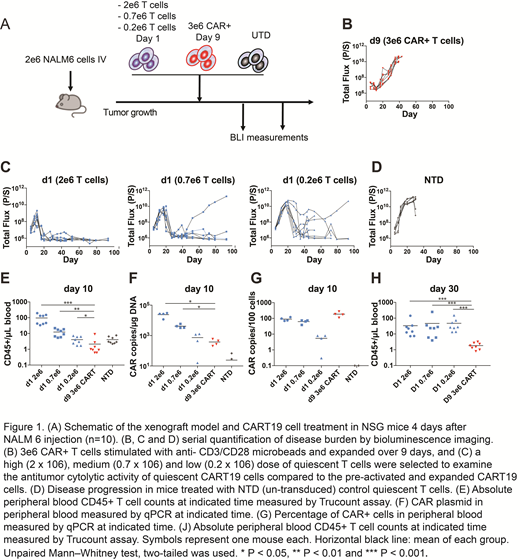
Here, we show that functional CD19-specific CAR T cells (CART19) can be generated in as little as 24 hours using lentiviral vectors without the need for prior T cell activation. We showed using a non-optimized process that a mean of 6.5% (range 2%-10%) of freshly isolated quiescent T cells can be transduced using an infrared red fluorescent protein (iRFP)-expressing lentiviral vector with slower kinetics of expression compared with activated T cell transduction (peak at 96 hrs vs. 48 hrs for quiescent and activated T cells, respectively). Although substantially less efficient compared to activated T cells, transduction was detected across all T cells subsets with central memory T cells showing the greatest transduction efficiency with a mean of 4-fold greater transduction compared with naïve T cells. Somewhat unexpectedly, CART19 cells generated from quiescent T cells using a CD19-specific CAR vector showed a 3-5 fold greater transgene expression compared with iRFP vectors transduced at similar MOI. However, we show that CAR expression can occur in quiescent T cells even without reverse transcription or integrase function, so called "pseudotransduction". Importantly, we show that this CAR expression produces T cells with cytolytic activity and effector cytokine production in response to antigen that is similar to activated and transduced CAR T cells. Using the well-characterized Nalm6 model of acute lymphoblastic leukemia, we show that CART19 cells generated by transduction of quiescent T cells for 16 hours followed by washing to remove vector exhibit dose-dependent anti-leukemic activity that is durable with injection of as little as 2x105 total T cells. We estimate the latter to contain ~2x104 T cells with integrated lentiviral vector based upon transduction efficiency determined in studies using non-tumor bearing mice. (Fig 1).
In summary, our results support the need for further investigation of CAR T cells that are generated using engineering of quiescent T cells. Taking advantage of the ability of lentiviral vectors to transfer genes to quiescent T cells, the highly abbreviated and simplified manufacturing approach described here has the potential to enhance therapeutic potency while also substantially reducing the materials and labor costs associated with current manufacturing approaches that use activated and expanded T cells. In addition, the rapid nature of this manufacturing has the potential to extend the population of patients that may be treated with these therapies by shortening the interval between aphaeresis collection and re-infusion of CAR T cells, which prevents the treatment of some patients with rapidly progressive disease.
Ghassemi:Novartis: Patents & Royalties. Milone:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal