While survival for patients with acute myeloid leukemia (AML) has increased in recent years, relapse and treatment-related mortality remain a significant problem, especially in older patients. Although the use of gemtuzumab ozogamicin (GO) has validated the concept of antibody directed therapy for AML targeting CD33, complications from myelosuppression and opportunistic infections due to on-target toxicity to normal CD33+ hematopoietic cells have limited this approach. In addition, only a subset of patients derives long-term benefit from GO. Thus, to improve the outcomes of CD33-targeted immunotherapy, more potent drugs are needed such as CD33/CD3 bispecific T-cell Engagers (BiTEs), as are strategies to protect normal blood cells from the cytotoxic effects of these agents.
In this study, we assessed the in vivo activity of the clinically exploited CD33/CD3 BiTE AMG330 using our previously described MATCH mouse model (Haworth et al., MTM 2017), in which human CD34+ stem/progenitor cells (HSPCs) are transplanted into neonate mice. This model enables the in vivo maturation of human HSPCs to establish a fully reconstituted blood and immune system, and in particular functional T cells that permit the testing of BiTEs without risks of graft-versus-host disease. Since CD33 is expressed on normal myeloid cells, causing significant on-target/off-leukemia effects, we also investigated whether our previously validated CRISPR/Cas9-based strategy (Humbert et al., Leukemia 2019) protects hematopoiesis from AMG330 activity.
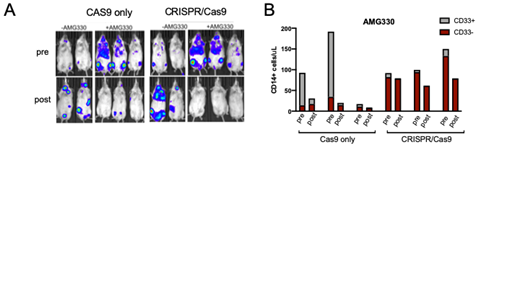
Mice were engrafted with mock-(Cas9 only) or CRISPR/Cas9-edited human CD34+ HSPCs to ablate CD33 expression. Normal multilineage engraftment was observed in both experimental groups, resulting in hematopoiesis that either expressed or almost entirely lacked CD33. Mice were also infused with AML tumor cells to validate AMG330 activity, which was administered intravenously to the animals for 5 consecutive days at 0.2mg/kg. AMG330 rapidly eliminated CD33+ Molm14 tumor cells within 5 days (Figure 1A) and reduced the number of circulating CD14+ monocytes in the mock group (Figure 1B), thus confirming the functionality of in vivo-matured autologous T-lymphocytes generated. In contrast, CRISPR/Cas9-edited cells were fully protected from AMG330 cytotoxicity, as evidenced by steady CD14+ counts pre- and post-treatment (Figure 1B). Together, these results validate the MATCH model for testing the efficacy and toxicity of CD33-directed immunotherapies, in particular novel BiTEs. In addition, the combination of HSPC protection and improved CD33 targeting to more effectively kill AML should be of great value for the development of novel BiTEs and other bispecific antibodies for the treatment of AML.
Figure 1: A) Leukemia burden measured by luciferase expression in mice from the two experimental groups treated or not with AMG330. B) Quantification of CD14+/CD33+ and CD14+/CD33− cells from peripheral blood of AMG330-treated animals in Cas9 only (n=3) and CRISPR/Cas9 (n = 3) groups. In A) and B), 'pre' is before AMG330 treatment and 'post' is 5 days after administration.
Walter:New Link Genetics: Consultancy; Argenx BVBA: Consultancy; Astellas: Consultancy; BioLineRx: Consultancy; BiVictriX: Consultancy; Amphivena Therapeutics: Consultancy, Equity Ownership; Boehringer Ingelheim: Consultancy; Aptevo Therapeutics: Consultancy, Research Funding; Boston Biomedical: Consultancy; Covagen: Consultancy; Daiichi Sankyo: Consultancy; Jazz Pharmaceuticals: Consultancy; Kite Pharma: Consultancy; Pfizer: Consultancy, Research Funding; Race Oncology: Consultancy; Seattle Genetics: Research Funding; Agios: Consultancy; Amgen: Consultancy. Kiem:Magenta Therapeutics: Consultancy; Rocket Pharma: Consultancy, Equity Ownership; Homology Medicines: Consultancy, Equity Ownership; CSL Behring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal