The therapeutic benefits of allogeneic hematopoietic stem cell transplantation (allo-HSCT) are primarily derived from graft-versus-leukemia (GvL) that is mediated by mature T cells in donor grafts. Unfortunately, graft-versus-host disease (GvHD) results from immune reactions of these same donor T cells against host tissues and organs after allo-HSCT. Due to this strong association between GvHD and the beneficial GvL, our lab and others have been working on developing effective therapeutic strategies to selectively prevent and treat GvHD without abrogating GvL.
Recently we have reported that pharmacologic inhibition of Janus kinases 1/2 (JAK1/JAK2) prevents GvHD by blocking IFNGR and IL6R signaling. Although baricitinib (BARI) and ruxolitinib (RUX) are both JAK1/JAK2 inhibitors with similar potency, BARI was found to be superior to RUX in prevention of GvHD in our mouse models of allo-HSCT. One of our proposed mechanistic pathways by which BARI prevents GvHD is via a robust increase in regulatory T cells (Tregs) which play a pivotal role in controlling GvHD. Since the proliferation and survival of Tregs are mediated through the IL2-JAK1/JAK3-STAT5 signaling pathway and that RUX is a more JAK3 inhibitor, we hypothesized that sparing JAK3 by BARI is critical for this increase of Tregs. To test this hypothesis, we administered BARI to allo-HSCT recipient mice with or without JAK3-specific inhibitor and monitored them for GvHD signs. The mice treated with BARI and iJAK3 had significantly lower Tregs and overall survival rates compared to the mice treated with BARI alone. This data suggests that BARI prevents GvHD by retaining JAK3, thereby increasing Tregs.
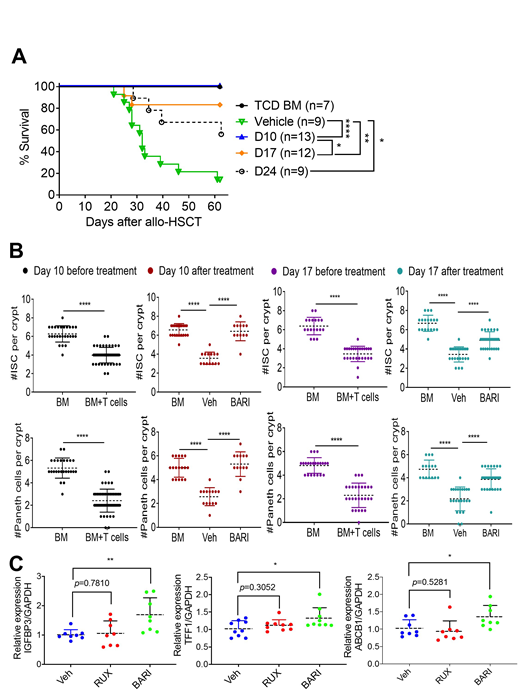
We have also previously reported that BARI treats established GvHD. However, the mechanisms by which BARI reverses ongoing GvHD remain unclear. Consequently, we hypothesized that BARI not only modulates immune cell functions as we previously reported, but also promotes restoration of GvHD damaged tissues. To test our hypothesis, we delayed the administration of BARI until mice developed clinically apparent GvHD and treated them starting on days 10, 17, or 24 after allo-HSCT. Despite this latency, the day 10 group showed significantly better overall survival (p<0.001, n=14) and lower histopathological grades (p<0.001, n=5) in comparison with all other groups (Fig. A).
Since the gastrointestinal (GI) tract is the one of most frequently diagnosed and severely damaged GvHD target organs, we tested the possible mechanistic pathways by which BARI reverses GvHD in the GI tract. We found that Paneth cells and intestinal stem cells which are both involved in maintaining the intestinal barrier integrity are substantially increased in day 10 (p<0.0001, n=13) and 17 (p<0.001, n=29) groups compared to the vehicle control (Fig. B). To understand the molecular mechanisms underlying BARI-mediated reversal of established GvHD, we treated human intestinal organoids with BARI and RUX. Upon observing an increased proliferation in organoids treated with BARI and RUX, we then performed RNA-seq analyses on them to determine the specific genes that are differentially regulated by the treatment of BARI and RUX. Thirteen genes were found to be significantly upregulated in the RUX and BARI treated samples and three of these genes (TFF1, ABCB1, and IGFBP3) have been shown to be positively associated with EGFR signaling while negatively associated with TNFR signaling. Once we confirmed the expression levels of these genes in the human organoids using real-time PCR, we then generated an in vitro established GvHD model by treating mouse intestinal organoids with inflammatory cytokines (IFNγ, TNFα, and IL-1β). Two days later, we treated these organoids with BARI and RUX to evaluate expressions of IGFBP3, TFF1 and ABCB1 (Fig. C). We found that BARI significantly upregulates expression of TFF1, ABCB1, and IGFBP3 RNA levels in those cytokine damaged intestinal organoids compared to RUX. Collectively, these data suggest that BARI possibly reverses established GvHD by promoting damaged tissue repair by activating EGFR signaling while inhibiting inflammation and crypt apoptosis by TNFR1 signaling through TFF1, IGFBP3, and ABCB1. Lastly, the increase of Tregs by sparing JAK3 and the upregulation of these three genes in BARI-treated groups may explain the superiority of BARI over RUX in both prevention and treatment of GvHD.
DiPersio:Karyopharm Therapeutics: Consultancy; RiverVest Venture Partners Arch Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; Incyte: Consultancy, Research Funding; Macrogenics: Research Funding, Speakers Bureau; WUGEN: Equity Ownership, Patents & Royalties, Research Funding; Magenta Therapeutics: Equity Ownership; Cellworks Group, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline Rx: Research Funding, Speakers Bureau; NeoImmune Tech: Research Funding. Choi:Daewoong Pharmaceuticals Co., Ltd: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal